正在加载图片...
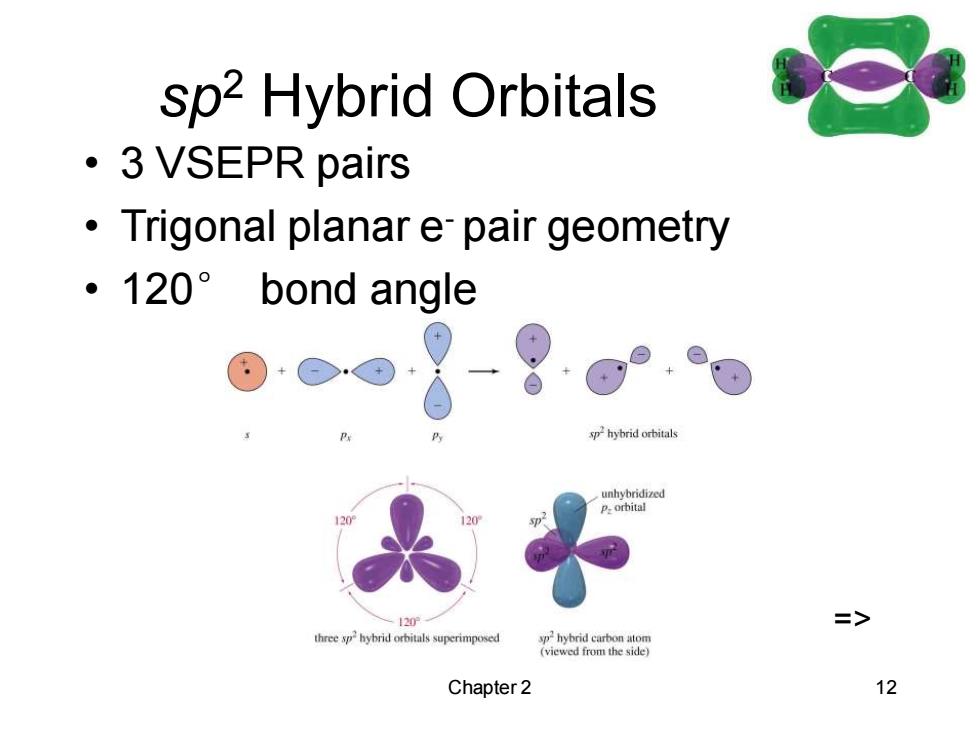
sp2 Hybrid Orbitals ·3 VSEPR pairs Trigonal planar e pair geometry ·120°bond angle 0w-g sphybrid orbitals .unhybridized P.orhital 120P => three sphybrid orbitals superimposed sphybrid carbon atom (viewed from the side) Chapter 2 12 Chapter 2 12 sp2 Hybrid Orbitals • 3 VSEPR pairs • Trigonal planar e- pair geometry • 120° bond angle =>