正在加载图片...
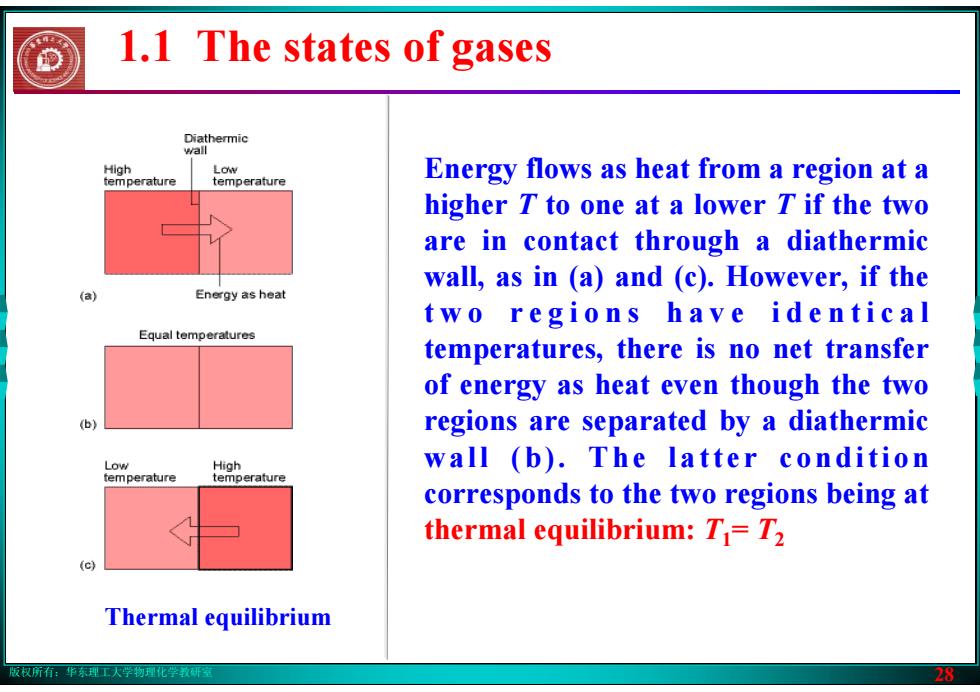
版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases版权所有:华东理工大学物理化学教研室 28 Thermal equilibrium Energy flows as heat from a region at a higher T to one at a lower T if the two are in contact through a diathermic wall, as in (a) and (c). However, if the t w o r e g i o n s h a v e i d e n t i c a l temperatures, there is no net transfer of energy as heat even though the two regions are separated by a diathermic wall (b). The latter condition corresponds to the two regions being at thermal equilibrium: T1= T2 1.1 The states of gases