正在加载图片...
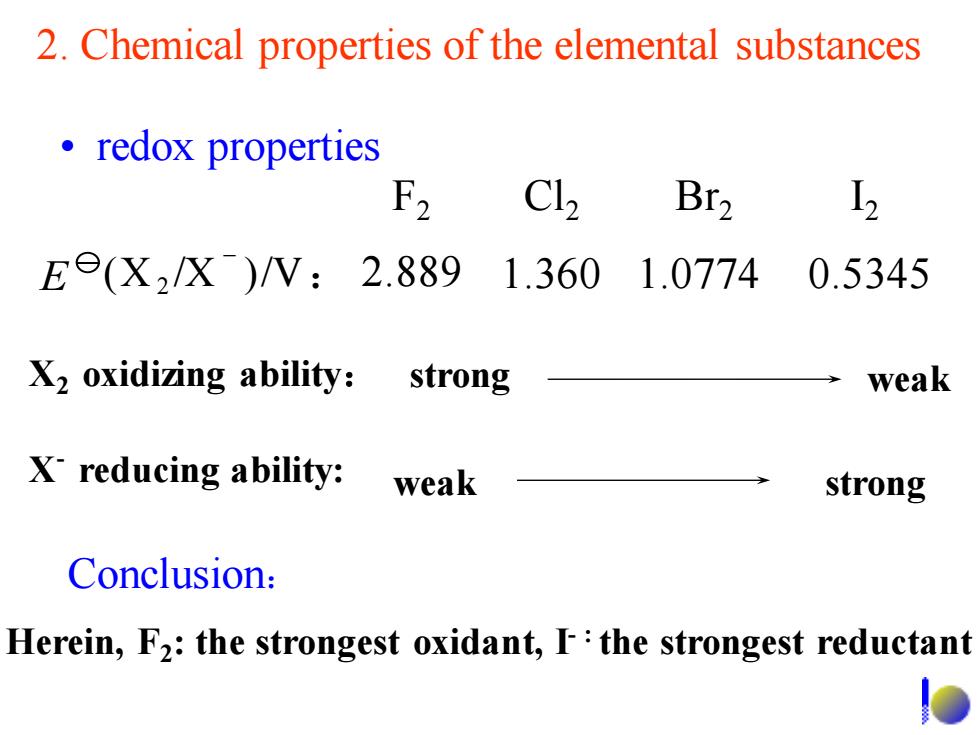
2.Chemical properties of the elemental substances ·redox properties F2 C12 Br2 I, Ee(X2/X)/V:2.8891.360 1.0774 0.5345 X2 oxidizing ability:strong weak X reducing ability: weak strong Conclusion: Herein,F2:the strongest oxidant,F:the strongest reductantF2 Cl2 Br2 I2 X2 oxidizing ability: strong weak X - reducing ability: • redox properties Herein, F2 : the strongest oxidant, I- : the strongest reductant 2. Chemical properties of the elemental substances (X 2 /X )/V: 2.889 1.360 1.0774 0.5345 - E Conclusion: weak strong