正在加载图片...
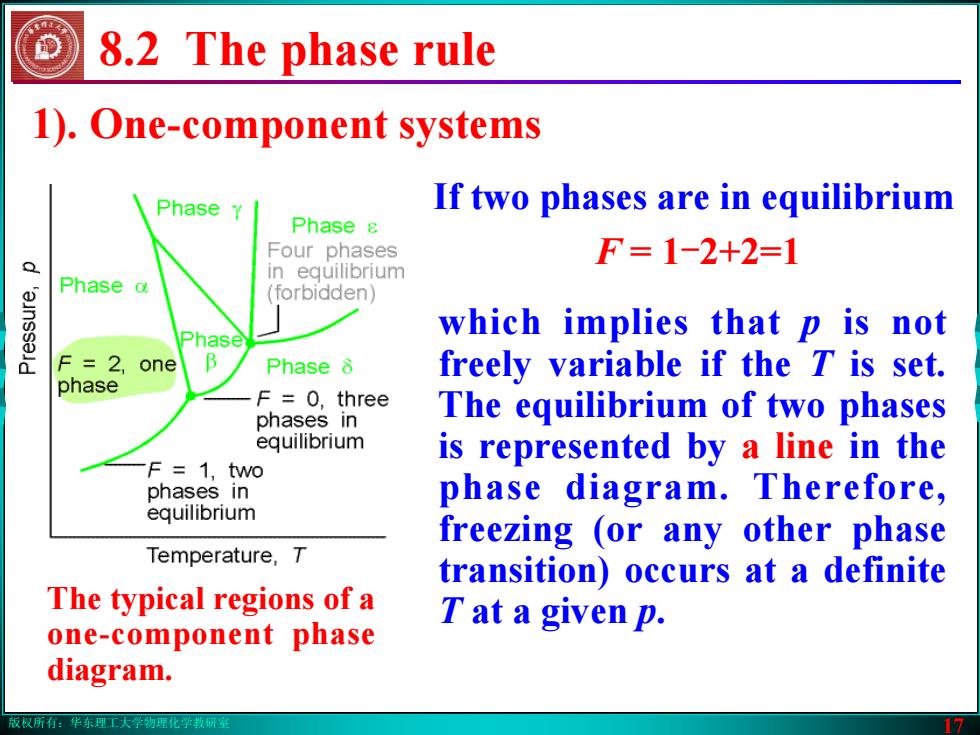
版权所有:华东理工大学物理化学教研室 17 1). One-component systems 8.2 The phase rule which implies that p is not freely variable if the T is set. The equilibrium of two phases is represented by a line in the phase diagram. Therefore, freezing (or any other phase transition) occurs at a definite T at a given p. If two phases are in equilibrium F = 1-2+2=1 The typical regions of a one-component phase diagram.版权所有:华东理工大学物理化学教研室 17 1). One-component systems 8.2 The phase rule which implies that p is not freely variable if the T is set. The equilibrium of two phases is represented by a line in the phase diagram. Therefore, freezing (or any other phase transition) occurs at a definite T at a given p. If two phases are in equilibrium F = 1-2+2=1 The typical regions of a one-component phase diagram