正在加载图片...
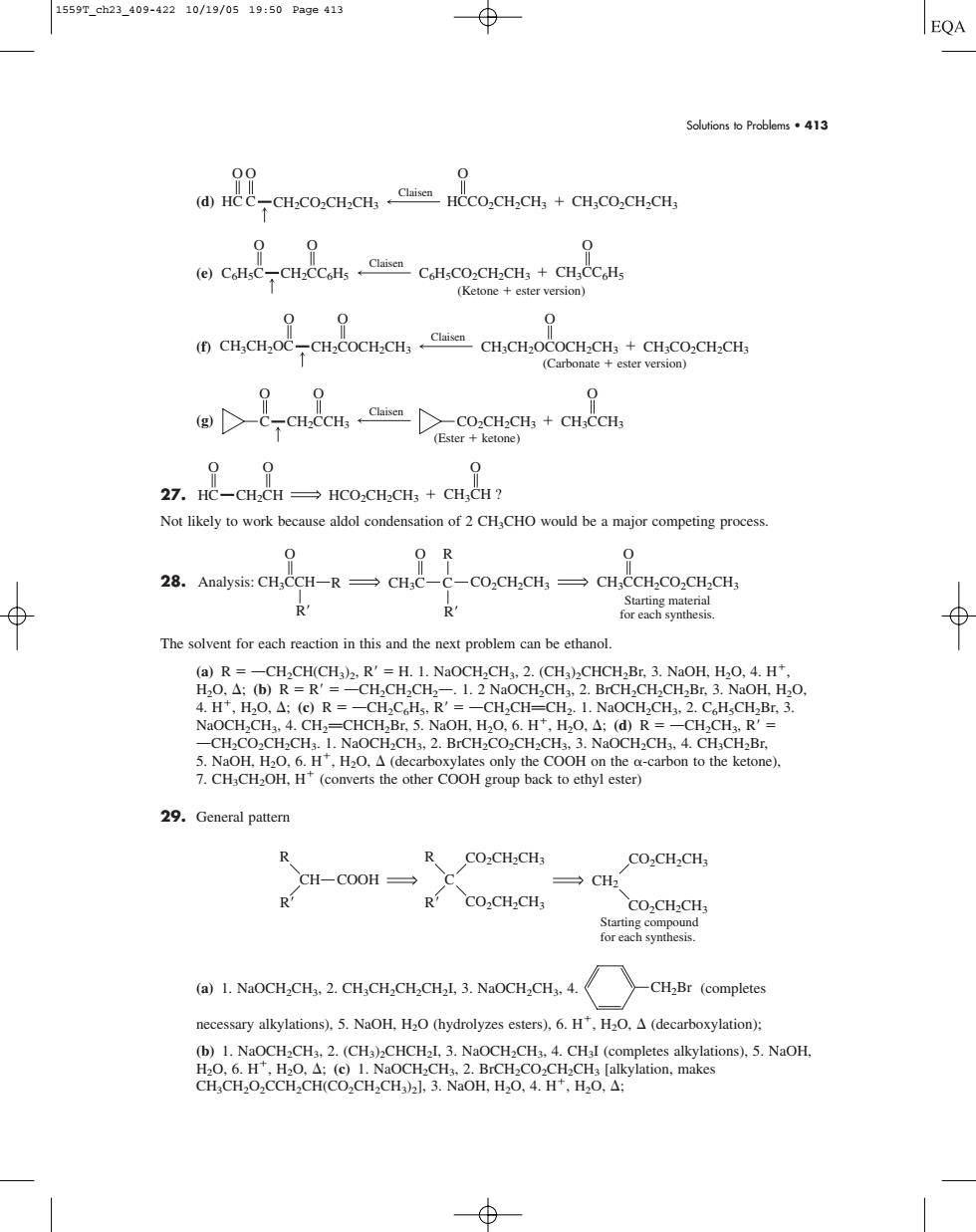
1559T_ch23_409-42210/19/0519:50Pa9e413 EQA Soutionso Problems413 ()HCCCH.CO.CH.CH,HcCO.CH.CH,+CH.CO.CH.CH, 0 CaeCCHtCllCo.CHC+cHcC (Ketone ester version) 0 CH.CH.OCCH.COCH.CH,CH.CH.OCOCH.CH.+CH.CO.CH.CH (Carbonate ester version 00 (,co.CH.CH,+CH. (Ester ketone) 00 27.HC-CH-CH HCO-CH-CH:CH:CH? Not likely to work because aldol condensation of 2 CHCHO would be a major competing process 0 O R 28.Analysis:CH,CCH-R CH;C- -CO.CH.CH,CH.CCH.CO.CH.CH3 R The solvent for each reaction in this and the next problem can be ethanol (a)R--CHCH(CH)2 R'-H.1.NaOCH,CH.2.(CH)CHCH,Br.3.NaOH,H.O,4.H" R=-CHCHR CH. CH.CO.CH,.NaOCH.CHs.2.BrCH.CO.CH.CH.3.NaOCH.CHs.4.CH,CH,Br. 29.General pattern CH-COOR 、CO2CH,CH3 CO2CH2CH, CH2 R CO2CH-CH; CO.CH-CH ()1.NaOCH.CH,2.CH,CH.CH.CHl.3.NaOCH,CH. 》-CHBr(completes necessary alkylations).5.NaOH.H2O(hydrolyzes esters).6.H.HaO.A(decarboxylation): 茶器 Solutions to Problems • 413 (d) (e) (f) (g) 27. Not likely to work because aldol condensation of 2 CH3CHO would be a major competing process. 28. The solvent for each reaction in this and the next problem can be ethanol. (a) R OCH2CH(CH3)2, R H. 1. NaOCH2CH3, 2. (CH3)2CHCH2Br, 3. NaOH, H2O, 4. H, H2O, ; (b) R R OCH2CH2CH2O. 1. 2 NaOCH2CH3, 2. BrCH2CH2CH2Br, 3. NaOH, H2O, 4. H, H2O, ; (c) R OCH2C6H5, R OCH2CHPCH2. 1. NaOCH2CH3, 2. C6H5CH2Br, 3. NaOCH2CH3, 4. CH2PCHCH2Br, 5. NaOH, H2O, 6. H, H2O, ; (d) R OCH2CH3, R OCH2CO2CH2CH3. 1. NaOCH2CH3, 2. BrCH2CO2CH2CH3, 3. NaOCH2CH3, 4. CH3CH2Br, 5. NaOH, H2O, 6. H, H2O, (decarboxylates only the COOH on the -carbon to the ketone), 7. CH3CH2OH, H (converts the other COOH group back to ethyl ester) 29. General pattern (a) 1. NaOCH2CH3, 2. CH3CH2CH2CH2I, 3. NaOCH2CH3, 4. (completes necessary alkylations), 5. NaOH, H2O (hydrolyzes esters), 6. H, H2O, (decarboxylation); (b) 1. NaOCH2CH3, 2. (CH3)2CHCH2I, 3. NaOCH2CH3, 4. CH3I (completes alkylations), 5. NaOH, H2O, 6. H, H2O, ; (c) 1. NaOCH2CH3, 2. BrCH2CO2CH2CH3 [alkylation, makes CH3CH2O2CCH2CH(CO2CH2CH3)2], 3. NaOH, H2O, 4. H, H2O, ; CH2Br CH COOH R R C R R CO2CH2CH3 CH2 CO2CH2CH3 CO2CH2CH3 CO2CH2CH3 Starting compound for each synthesis. Analysis: CH3CCH CH3C C CO2CH2CH3 CH3CCH2CO2CH2CH3 O R R R O O Starting material R for each synthesis. HC CH2CH HCO2CH2CH3 CH3CH ? O O O (Ester ketone) C CH2CCH3 CO2CH2CH3 CH3CCH3 O O O Claisen Claisen CH3CH2OC CH2COCH2CH3 O O (Carbonate ester version) CH3CH2OCOCH2CH3 CH3CO2CH2CH3 O Claisen CH3CC6H5 (Ketone ester version) C6H5C C CH2CC6H5 6H5CO2CH2CH3 O O O Claisen HC C CH2CO2CH2CH3 HCCO2CH2CH3 CH3CO2CH2CH3 O O O 1559T_ch23_409-422 10/19/05 19:50 Page 413�����������������