正在加载图片...
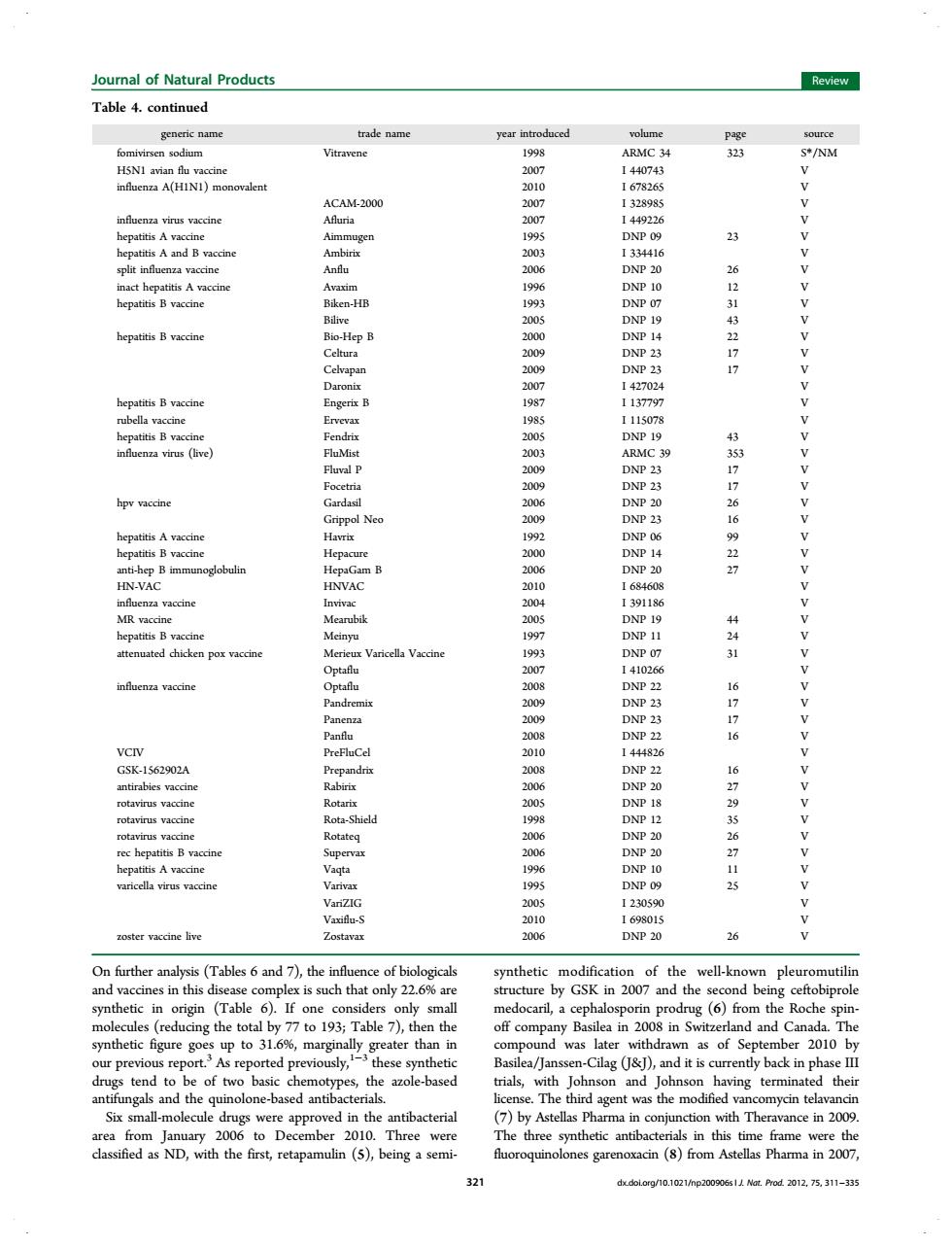
ournal of Natural Products Review Table 4.continued liihinaiinnsaia 20 23 Vaccin LLEEEEEELEEEEEEEEEEEEEEEELEEEEEEREEEEEEN 4216277 337766927 441 6776 167956713 (Tables 6 and 7),the hetic vell-kr pleu (Table 6)If 6 tal by 193 Table in Sv (&D tend to two basic ials, ohnson thei agent t wa the mo 6. 200 Three acterials in this time frame classified as ND,with the first,retapamulin (5),beingasemi- nes ga oxacin (8)from Astellas Pharma in 2007 On further analysis (Tables 6 and 7), the influence of biologicals and vaccines in this disease complex is such that only 22.6% are synthetic in origin (Table 6). If one considers only small molecules (reducing the total by 77 to 193; Table 7), then the synthetic figure goes up to 31.6%, marginally greater than in our previous report.3 As reported previously,1−3 these synthetic drugs tend to be of two basic chemotypes, the azole-based antifungals and the quinolone-based antibacterials. Six small-molecule drugs were approved in the antibacterial area from January 2006 to December 2010. Three were classified as ND, with the first, retapamulin (5), being a semisynthetic modification of the well-known pleuromutilin structure by GSK in 2007 and the second being ceftobiprole medocaril, a cephalosporin prodrug (6) from the Roche spinoff company Basilea in 2008 in Switzerland and Canada. The compound was later withdrawn as of September 2010 by Basilea/Janssen-Cilag (J&J), and it is currently back in phase III trials, with Johnson and Johnson having terminated their license. The third agent was the modified vancomycin telavancin (7) by Astellas Pharma in conjunction with Theravance in 2009. The three synthetic antibacterials in this time frame were the fluoroquinolones garenoxacin (8) from Astellas Pharma in 2007, Table 4. continued generic name trade name year introduced volume page source fomivirsen sodium Vitravene 1998 ARMC 34 323 S*/NM H5N1 avian flu vaccine 2007 I 440743 V influenza A(H1N1) monovalent 2010 I 678265 V ACAM-2000 2007 I 328985 V influenza virus vaccine Afluria 2007 I 449226 V hepatitis A vaccine Aimmugen 1995 DNP 09 23 V hepatitis A and B vaccine Ambirix 2003 I 334416 V split influenza vaccine Anflu 2006 DNP 20 26 V inact hepatitis A vaccine Avaxim 1996 DNP 10 12 V hepatitis B vaccine Biken-HB 1993 DNP 07 31 V Bilive 2005 DNP 19 43 V hepatitis B vaccine Bio-Hep B 2000 DNP 14 22 V Celtura 2009 DNP 23 17 V Celvapan 2009 DNP 23 17 V Daronix 2007 I 427024 V hepatitis B vaccine Engerix B 1987 I 137797 V rubella vaccine Ervevax 1985 I 115078 V hepatitis B vaccine Fendrix 2005 DNP 19 43 V influenza virus (live) FluMist 2003 ARMC 39 353 V Fluval P 2009 DNP 23 17 V Focetria 2009 DNP 23 17 V hpv vaccine Gardasil 2006 DNP 20 26 V Grippol Neo 2009 DNP 23 16 V hepatitis A vaccine Havrix 1992 DNP 06 99 V hepatitis B vaccine Hepacure 2000 DNP 14 22 V anti-hep B immunoglobulin HepaGam B 2006 DNP 20 27 V HN-VAC HNVAC 2010 I 684608 V influenza vaccine Invivac 2004 I 391186 V MR vaccine Mearubik 2005 DNP 19 44 V hepatitis B vaccine Meinyu 1997 DNP 11 24 V attenuated chicken pox vaccine Merieux Varicella Vaccine 1993 DNP 07 31 V Optaflu 2007 I 410266 V influenza vaccine Optaflu 2008 DNP 22 16 V Pandremix 2009 DNP 23 17 V Panenza 2009 DNP 23 17 V Panflu 2008 DNP 22 16 V VCIV PreFluCel 2010 I 444826 V GSK-1562902A Prepandrix 2008 DNP 22 16 V antirabies vaccine Rabirix 2006 DNP 20 27 V rotavirus vaccine Rotarix 2005 DNP 18 29 V rotavirus vaccine Rota-Shield 1998 DNP 12 35 V rotavirus vaccine Rotateq 2006 DNP 20 26 V rec hepatitis B vaccine Supervax 2006 DNP 20 27 V hepatitis A vaccine Vaqta 1996 DNP 10 11 V varicella virus vaccine Varivax 1995 DNP 09 25 V VariZIG 2005 I 230590 V Vaxiflu-S 2010 I 698015 V zoster vaccine live Zostavax 2006 DNP 20 26 V Journal of Natural Products Review 321 dx.doi.org/10.1021/np200906s | J. Nat. Prod. 2012, 75, 311−335