正在加载图片...
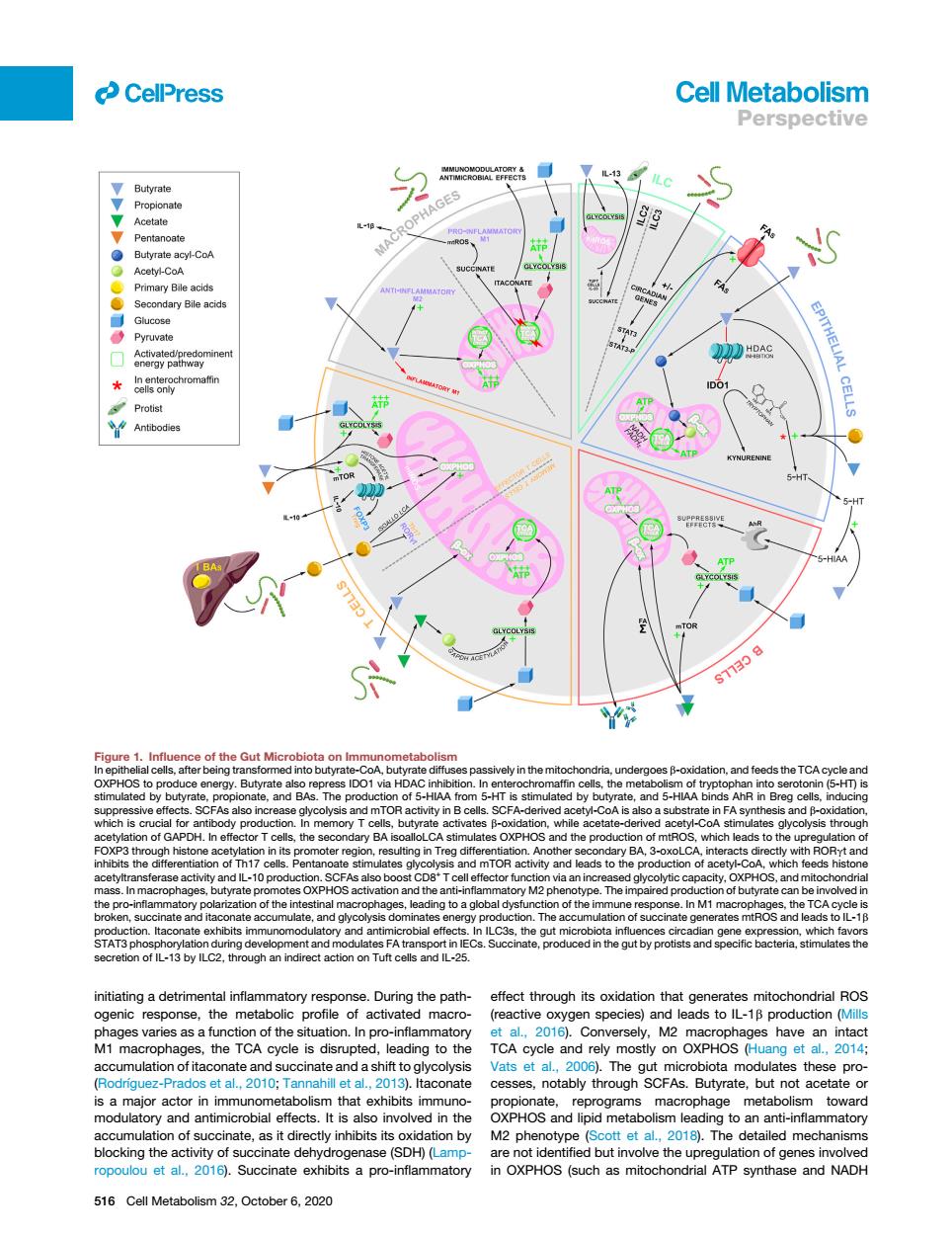
CellPress Cell Metabolism Perspective 2学 cetyt-CoA Protist ATP ot the Gut micr TA initiating a detrimental inflammatory response.During the path effect through its oxidation that generates mitochondrial ROS 】macf0 spec eaas to IL-1B production s.the TCA cycle is disrupted,leading to the TCA cycle and mostly on OXPHOS (Huang 2010T 201 taconate is a major actor in immunome bo ism that exhibits ir muno- reprograms macrophage vard n of suc ate as it di btsoxid M2 n (Scott et al 20 e(SDH)(Lamp are not ic d but involve the ropoulou et a 5 516 Cell Metabolism 2,October6,020initiating a detrimental inflammatory response. During the pathogenic response, the metabolic profile of activated macrophages varies as a function of the situation. In pro-inflammatory M1 macrophages, the TCA cycle is disrupted, leading to the accumulation of itaconate and succinate and a shift to glycolysis (Rodrı´guez-Prados et al., 2010; Tannahill et al., 2013). Itaconate is a major actor in immunometabolism that exhibits immunomodulatory and antimicrobial effects. It is also involved in the accumulation of succinate, as it directly inhibits its oxidation by blocking the activity of succinate dehydrogenase (SDH) (Lampropoulou et al., 2016). Succinate exhibits a pro-inflammatory effect through its oxidation that generates mitochondrial ROS (reactive oxygen species) and leads to IL-1b production (Mills et al., 2016). Conversely, M2 macrophages have an intact TCA cycle and rely mostly on OXPHOS (Huang et al., 2014; Vats et al., 2006). The gut microbiota modulates these processes, notably through SCFAs. Butyrate, but not acetate or propionate, reprograms macrophage metabolism toward OXPHOS and lipid metabolism leading to an anti-inflammatory M2 phenotype (Scott et al., 2018). The detailed mechanisms are not identified but involve the upregulation of genes involved in OXPHOS (such as mitochondrial ATP synthase and NADH Figure 1. Influence of the Gut Microbiota on Immunometabolism In epithelial cells, after being transformed into butyrate-CoA, butyrate diffuses passively in the mitochondria, undergoes b-oxidation, and feeds the TCA cycle and OXPHOS to produce energy. Butyrate also repress IDO1 via HDAC inhibition. In enterochromaffin cells, the metabolism of tryptophan into serotonin (5-HT) is stimulated by butyrate, propionate, and BAs. The production of 5-HIAA from 5-HT is stimulated by butyrate, and 5-HIAA binds AhR in Breg cells, inducing suppressive effects. SCFAs also increase glycolysis and mTOR activity in B cells. SCFA-derived acetyl-CoA is also a substrate in FA synthesis and b-oxidation, which is crucial for antibody production. In memory T cells, butyrate activates b-oxidation, while acetate-derived acetyl-CoA stimulates glycolysis through acetylation of GAPDH. In effector T cells, the secondary BA isoalloLCA stimulates OXPHOS and the production of mtROS, which leads to the upregulation of FOXP3 through histone acetylation in its promoter region, resulting in Treg differentiation. Another secondary BA, 3-oxoLCA, interacts directly with RORgt and inhibits the differentiation of Th17 cells. Pentanoate stimulates glycolysis and mTOR activity and leads to the production of acetyl-CoA, which feeds histone acetyltransferase activity and IL-10 production. SCFAs also boost CD8+ T cell effector function via an increased glycolytic capacity, OXPHOS, and mitochondrial mass. In macrophages, butyrate promotes OXPHOS activation and the anti-inflammatory M2 phenotype. The impaired production of butyrate can be involved in the pro-inflammatory polarization of the intestinal macrophages, leading to a global dysfunction of the immune response. In M1 macrophages, the TCA cycle is broken, succinate and itaconate accumulate, and glycolysis dominates energy production. The accumulation of succinate generates mtROS and leads to IL-1b production. Itaconate exhibits immunomodulatory and antimicrobial effects. In ILC3s, the gut microbiota influences circadian gene expression, which favors STAT3 phosphorylation during development and modulates FA transport in IECs. Succinate, produced in the gut by protists and specific bacteria, stimulates the secretion of IL-13 by ILC2, through an indirect action on Tuft cells and IL-25. ll 516 Cell Metabolism 32, October 6, 2020 Perspective