正在加载图片...
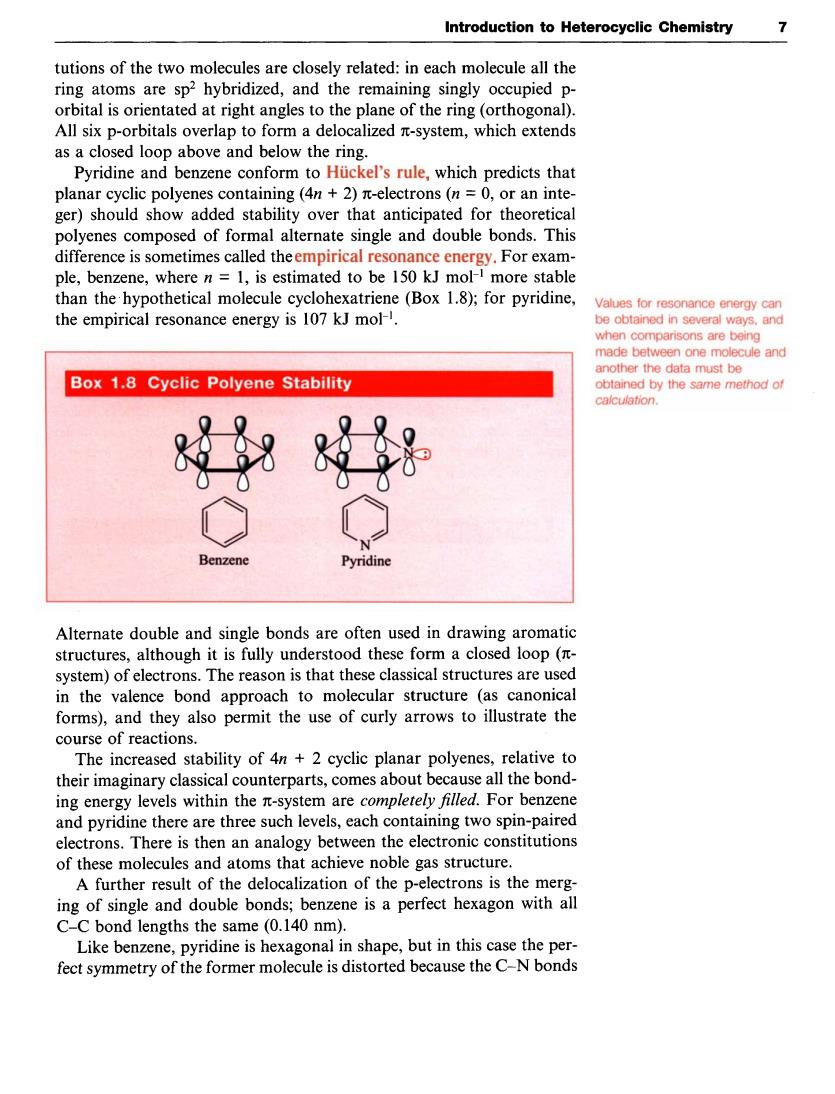
Introduction to Heterocyclic Chemistry 7 tutions of the two molecules are closely related:in each molecule all the ring atoms are sp2 hybridized,and the remaining singly occupied p- orbital is orientated at right angles to the plane of the ring(orthogonal). All six p-orbitals overlap to fo rm a delocalized -system,which extends as a closed loop above and below the ring. Pyridine and benzene conform to Huckel's rule,which predicts that planar cyclic polyenes containing(4n+2)n-electrons (n=0.or an inte- ger)should show added stability over that anticipated for theo retical polyenes composed of formal alternate single and double bonds.This difference is sometimes called theempirical resonance energy.For exam- ple.benzene.where n=1.is estimated to be 150 kJ mol-1 more stable than the hypothetical clohexatriene (Box 1.8):for pyridine Values for resonance energy can the empirical resonance energy is 107 kJ mol another the data must be Box 1.8 Cyclic Polyene Stability aned by the same methodo 8好} Benzene Alternate double and single bonds are often used in drawing aromatic structures,although it is fully understood these form a closed loop system)of electrons.The reason is that these classical structures are used in the valence bond approach to molecular structure (as canonical forms),and they also permit the use of curly arrows to illustrate the course of reactions. The increased stability of 4n+2 cyclic planar polyenes,relative to their imaginary classical counterparts,comes about because all the bond- electrons.There is then an analogy between the electronic constitutions of these molecules and atoms that achieve noble gas structure. A further result of the delocalization of the p-electrons is the merg. ing of single and double bonds;benzee is a perfect hexagon with all C-C bond lengths the same (0.140 nm). Like benzene,pyridine is hexagonal in shape,but in this case the per- fect t symmetry of the former molecule is distorted because the C-N bondsIntroduction to Heterocyclic Chemistry 7 tutions of the two molecules are closely related: in each molecule all the ring atoms are sp2 hybridized, and the remaining singly occupied porbital is orientated at right angles to the plane of the ring (orthogonal). All six p-orbitals overlap to form a delocalized n-system, which extends as a closed loop above and below the ring. Pyridine and benzene conform to Hiickel's rule, which predicts that planar cyclic polyenes containing (4n + 2) n-electrons (n = 0, or an integer) should show added stability over that anticipated for theoretical polyenes composed of formal alternate single and double bonds. This difference is sometimes called the empirical resonance energy. For example, benzene, where n = 1, is estimated to be 150 kJ mol-' more stable than the, hypothetical molecule cyclohexatriene (Box 1.8); for pyridine, Values for be obtained in several ways, and when comparisons are being made between one molecule and another the data must be obtained by the same method of calculation. energy can the empirical resonance energy is 107 kJ mol-'. Alternate double and single bonds are often used in drawing aromatic structures, although it is fully understood these form a closed loop (nsystem) of electrons. The reason is that these classical structures are used in the valence bond approach to molecular structure (as canonical forms), and they also permit the use of curly arrows to illustrate the course of reactions. The increased stability of 4n + 2 cyclic planar polyenes, relative to their imaginary classical counterparts, comes about because all the bonding energy levels within the n-system are completely filled. For benzene and pyridine there are three such levels, each containing two spin-paired electrons. There is then an analogy between the electronic constitutions of these molecules and atoms that achieve noble gas structure. A further result of the delocalization of the p-electrons is the merging of single and double bonds; benzene is a perfect hexagon with all C-C bond lengths the same (0.140 nm). Like benzene, pyridine is hexagonal in shape, but in this case the perfect symmetry of the former molecule is distorted because the C-N bonds