正在加载图片...
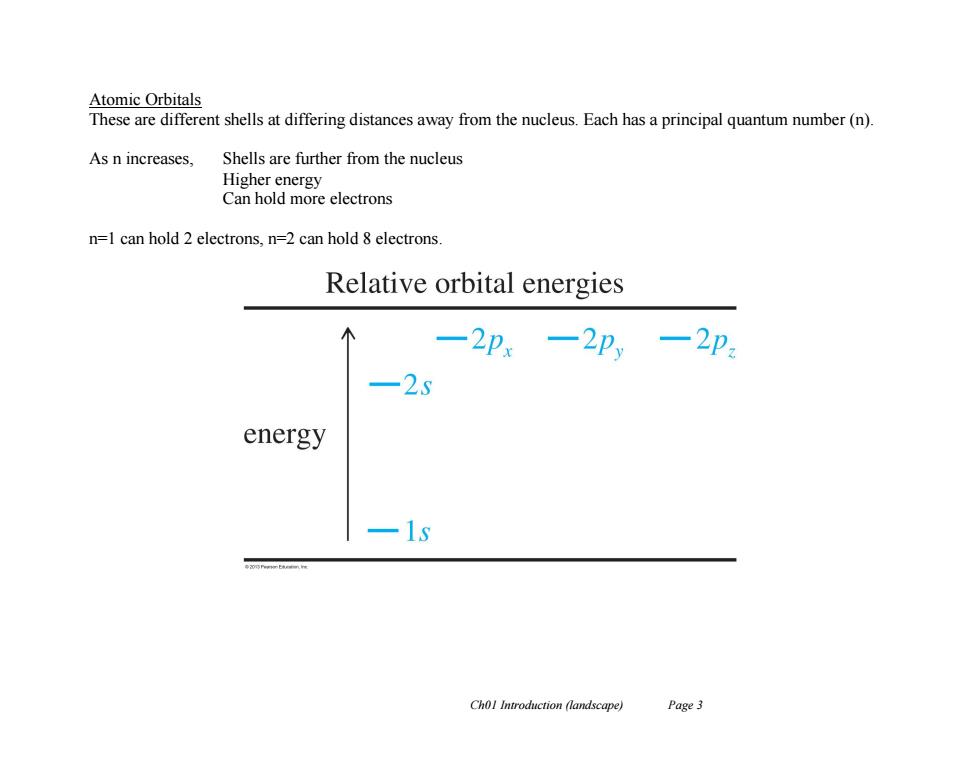
Atomic Orbitals These are different shells at differing distances away from the nucleus.Each has a principal quantum number(n). As n increases, Shells are further from the nucleus Higher energy Can hold more electrons n=1 can hold 2 electrons,n=2 can hold 8 electrons. Relative orbital energies 一2p.一2p,一2p 25 energy 1g Chol Introduction (landscape) Page3 Ch01 Introduction (landscape) Page 3 Atomic Orbitals These are different shells at differing distances away from the nucleus. Each has a principal quantum number (n). As n increases, Shells are further from the nucleus Higher energy Can hold more electrons n=1 can hold 2 electrons, n=2 can hold 8 electrons