正在加载图片...
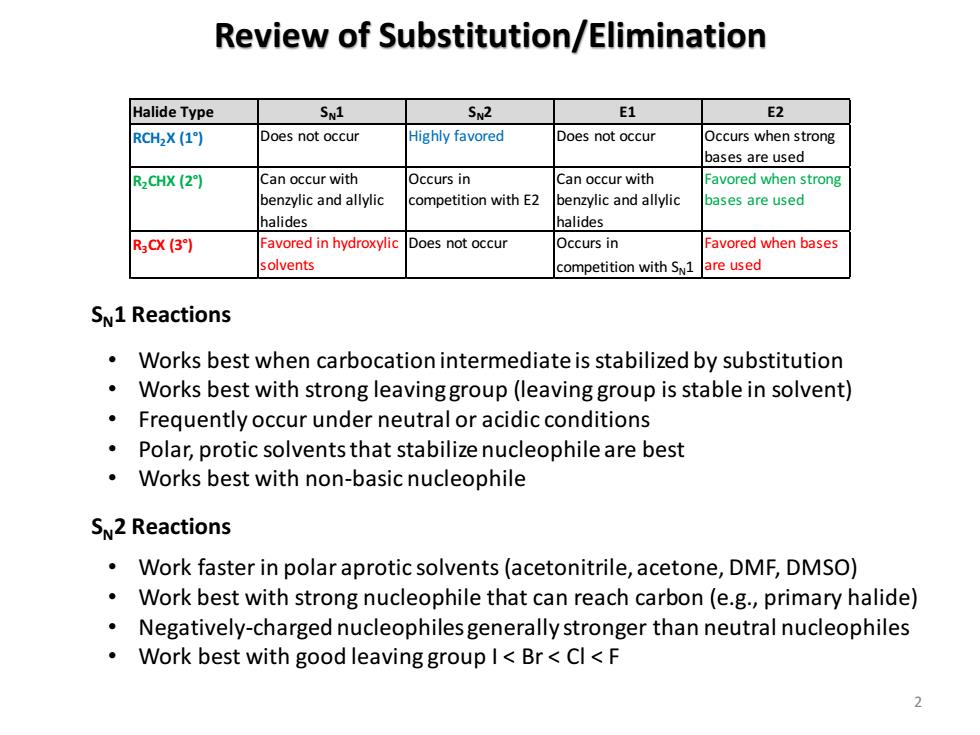
Review of Substitution/Elimination Halide Type SN1 SN2 E1 E2 RCH2X (1) Does not occur Highly favored Does not occur Occurs when strong bases are used R2CHX (2) Can occur with Occurs in Can occur with Favored when strong benzylic and allylic competition with E2 benzylic and allylic bases are used halides halides R3CX(3】 Favored in hydroxylic Does not occur Occurs in Favored when bases solvents competition with S1 are used SN1 Reactions Works best when carbocation intermediate is stabilized by substitution Works best with strong leavinggroup(leaving group is stable in solvent) Frequently occur under neutral or acidic conditions Polar,protic solvents that stabilize nucleophile are best Works best with non-basic nucleophile SN2 Reactions Work faster in polar aprotic solvents(acetonitrile,acetone,DMF,DMSO) Work best with strong nucleophile that can reach carbon(e.g.,primary halide) Negatively-charged nucleophiles generally stronger than neutral nucleophiles Work best with good leaving group I<Br<Cl<F Review of Substitution/Elimination 2 Halide Type SN1 SN2 E1 E2 RCH2X (1°) Does not occur Highly favored Does not occur Occurs when strong bases are used R2CHX (2°) Can occur with benzylic and allylic halides Occurs in competition with E2 Can occur with benzylic and allylic halides Favored when strong bases are used R3CX (3°) Favored in hydroxylic solvents Does not occur Occurs in competition with SN1 Favored when bases are used SN1 Reactions SN2 Reactions • Works best when carbocation intermediate is stabilized by substitution • Works best with strong leaving group (leaving group is stable in solvent) • Frequently occur under neutral or acidic conditions • Polar, protic solvents that stabilize nucleophile are best • Works best with non-basic nucleophile • Work faster in polar aprotic solvents (acetonitrile, acetone, DMF, DMSO) • Work best with strong nucleophile that can reach carbon (e.g., primary halide) • Negatively-charged nucleophiles generally stronger than neutral nucleophiles • Work best with good leaving group I < Br < Cl < F