正在加载图片...
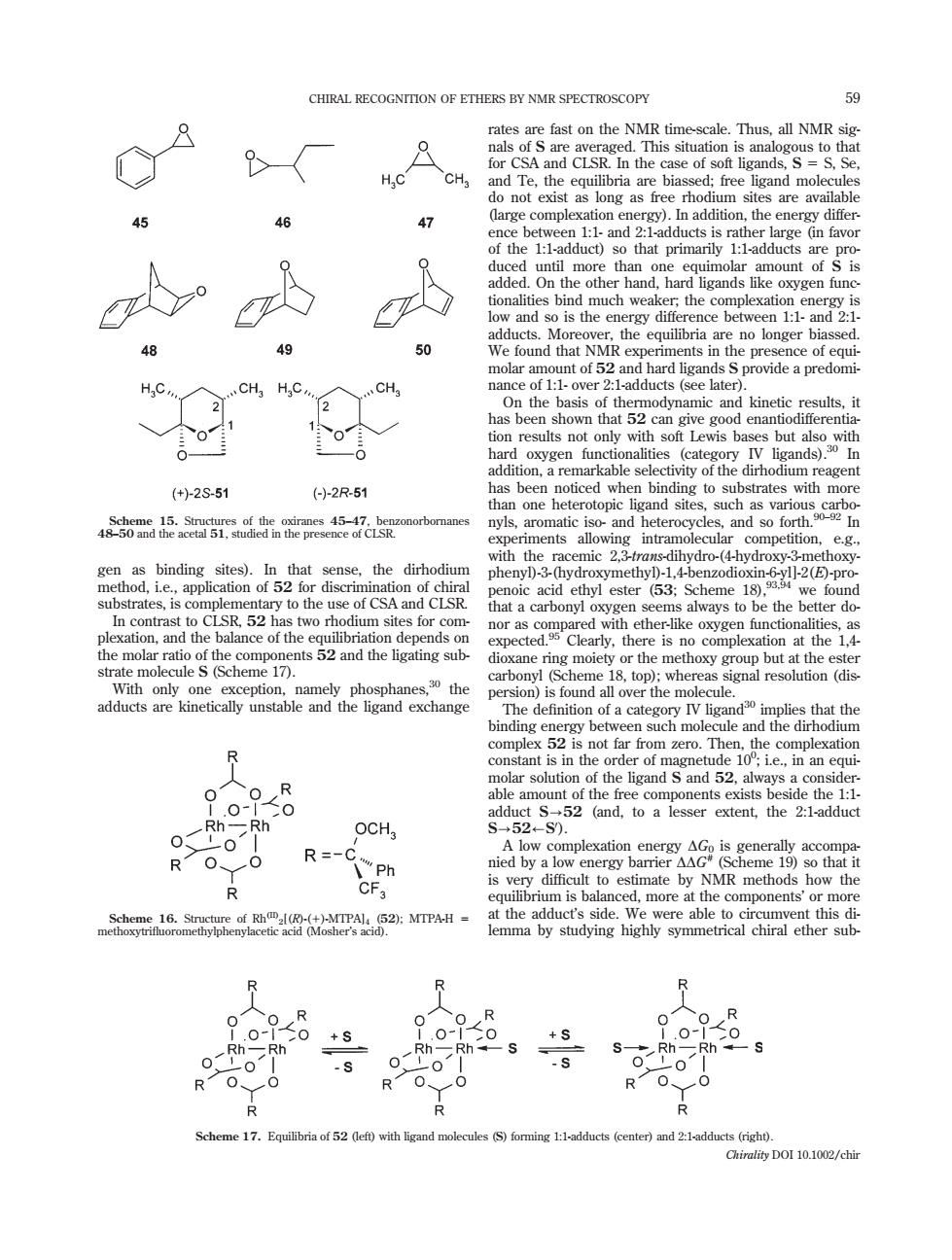
CHIRAL RECOGNITION OF ETHERS BY NMR SPECTROSCOPY 9 nd Te.the equilibria xationg as 47 intil more than hard mount of nalitie bind much wea plexation Mo is the en n 1:1-and 2. 49 50 ound that NMR HC. CH HC ults not owith sof Lewis ba but (+)-2S51 ()2R-51 ore nyls, and and so fo ththe race how e the be ter do o complexation molecule S(Scheme 17). ule that the bind ng betwee n such mo R ar solu the ligand S and 52 the 1 52 (and.to a lesser extent,the 2:1-adduct OCH R=-0 nied by a NMR 19 n is b e components' na16SaR图C52 )MTPAH- this lemma by studving highly symmetical chiral ether sub 0+s R 0 +S Rh- :S- Rh -S Scheme 17.Equili of 52 (left) )and 2:1adducts (right Chirality DOI 10.1002/chingen as binding sites). In that sense, the dirhodium method, i.e., application of 52 for discrimination of chiral substrates, is complementary to the use of CSA and CLSR. In contrast to CLSR, 52 has two rhodium sites for complexation, and the balance of the equilibriation depends on the molar ratio of the components 52 and the ligating substrate molecule S (Scheme 17). With only one exception, namely phosphanes,30 the adducts are kinetically unstable and the ligand exchange rates are fast on the NMR time-scale. Thus, all NMR signals of S are averaged. This situation is analogous to that for CSA and CLSR. In the case of soft ligands, S 5 S, Se, and Te, the equilibria are biassed; free ligand molecules do not exist as long as free rhodium sites are available (large complexation energy). In addition, the energy difference between 1:1- and 2:1-adducts is rather large (in favor of the 1:1-adduct) so that primarily 1:1-adducts are produced until more than one equimolar amount of S is added. On the other hand, hard ligands like oxygen functionalities bind much weaker; the complexation energy is low and so is the energy difference between 1:1- and 2:1- adducts. Moreover, the equilibria are no longer biassed. We found that NMR experiments in the presence of equimolar amount of 52 and hard ligands S provide a predominance of 1:1- over 2:1-adducts (see later). On the basis of thermodynamic and kinetic results, it has been shown that 52 can give good enantiodifferentiation results not only with soft Lewis bases but also with hard oxygen functionalities (category IV ligands).30 In addition, a remarkable selectivity of the dirhodium reagent has been noticed when binding to substrates with more than one heterotopic ligand sites, such as various carbonyls, aromatic iso- and heterocycles, and so forth.90–92 In experiments allowing intramolecular competition, e.g., with the racemic 2,3-trans-dihydro-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-1,4-benzodioxin-6-yl]-2(E)-propenoic acid ethyl ester (53; Scheme 18),93,94 we found that a carbonyl oxygen seems always to be the better donor as compared with ether-like oxygen functionalities, as expected.95 Clearly, there is no complexation at the 1,4- dioxane ring moiety or the methoxy group but at the ester carbonyl (Scheme 18, top); whereas signal resolution (dispersion) is found all over the molecule. The definition of a category IV ligand30 implies that the binding energy between such molecule and the dirhodium complex 52 is not far from zero. Then, the complexation constant is in the order of magnetude 100 ; i.e., in an equimolar solution of the ligand S and 52, always a considerable amount of the free components exists beside the 1:1- adduct S?52 (and, to a lesser extent, the 2:1-adduct S?52/S0 ). A low complexation energy DG0 is generally accompanied by a low energy barrier DDG# (Scheme 19) so that it is very difficult to estimate by NMR methods how the equilibrium is balanced, more at the components’ or more at the adduct’s side. We were able to circumvent this dilemma by studying highly symmetrical chiral ether subScheme 15. Structures of the oxiranes 45–47, benzonorbornanes 48–50 and the acetal 51, studied in the presence of CLSR. Scheme 16. Structure of Rh(II)2[(R)-(1)-MTPA]4 (52); MTPA-H 5 methoxytrifluoromethylphenylacetic acid (Mosher’s acid). Scheme 17. Equilibria of 52 (left) with ligand molecules (S) forming 1:1-adducts (center) and 2:1-adducts (right). CHIRAL RECOGNITION OF ETHERS BY NMR SPECTROSCOPY 59 Chirality DOI 10.1002/chir