正在加载图片...
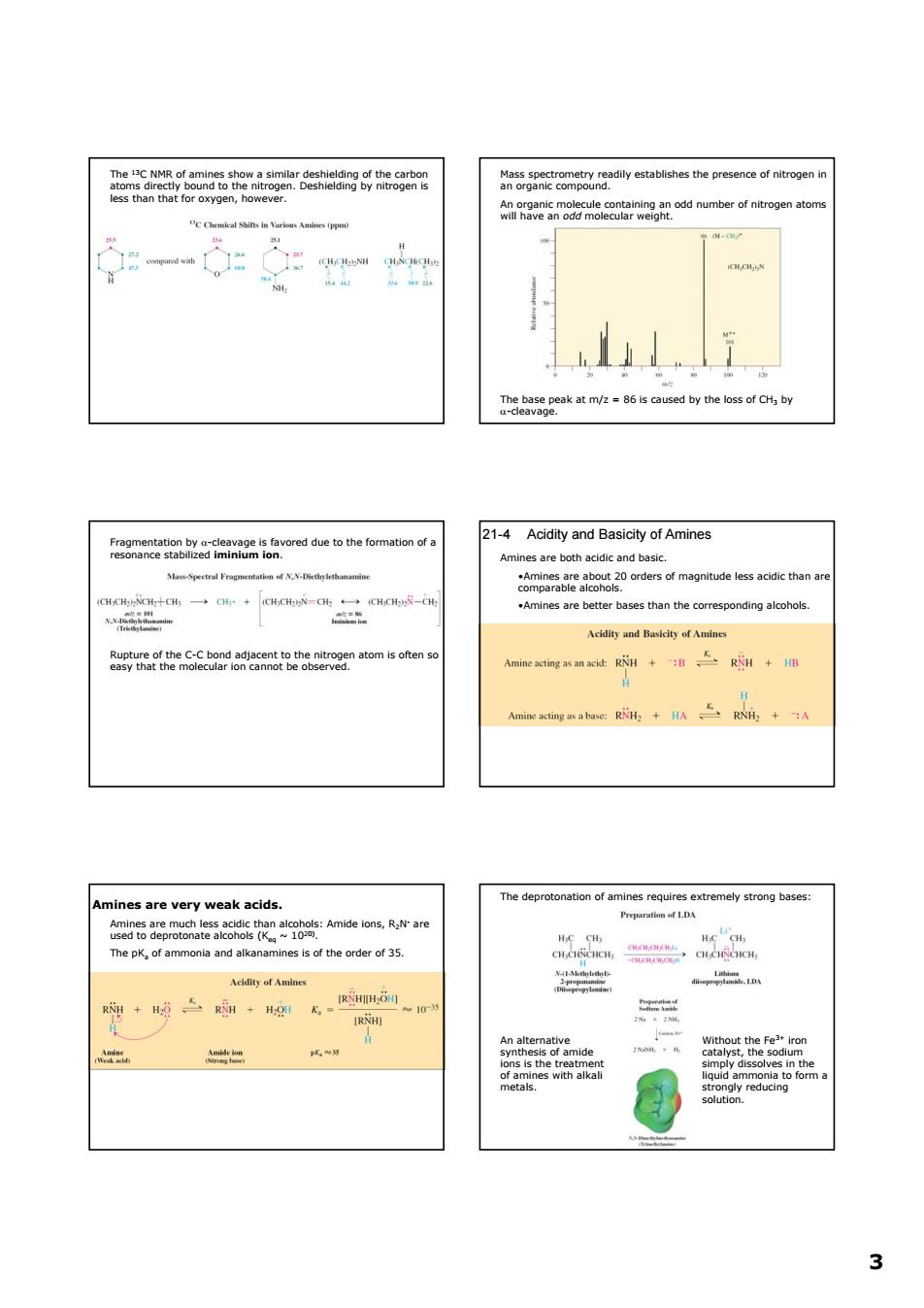
三2eae 8hesgompygayesablshesthepresenceotnitroe e8miy-anaeoaorddetothe1omatomoda 21-4 Acidity and Basicity of Amines Amines are both acidic and basic. Mene-Specte sf N. Acidity and Basicity of Amines gecfh地enSCtaaeamteeoeteagnatomsotenq es are ver y weak acids me pKof alkanaminess of the order of 35. R 3 3 The 13C NMR of amines show a similar deshielding of the carbon atoms directly bound to the nitrogen. Deshielding by nitrogen is less than that for oxygen, however. Mass spectrometry readily establishes the presence of nitrogen in an organic compound. An organic molecule containing an odd number of nitrogen atoms will have an odd molecular weight. The base peak at m/z = 86 is caused by the loss of CH3 by α-cleavage. Fragmentation by α-cleavage is favored due to the formation of a resonance stabilized iminium ion. Rupture of the C-C bond adjacent to the nitrogen atom is often so easy that the molecular ion cannot be observed. 21-4 Acidity and Basicity of Amines Amines are both acidic and basic. •Amines are about 20 orders of magnitude less acidic than are comparable alcohols. •Amines are better bases than the corresponding alcohols. Amines are very weak acids. Amines are much less acidic than alcohols: Amide ions, R2N- are used to deprotonate alcohols (Keq ~ 1020). The pKa of ammonia and alkanamines is of the order of 35. The deprotonation of amines requires extremely strong bases: An alternative synthesis of amide ions is the treatment of amines with alkali metals. Without the Fe3+ iron catalyst, the sodium simply dissolves in the liquid ammonia to form a strongly reducing solution