正在加载图片...
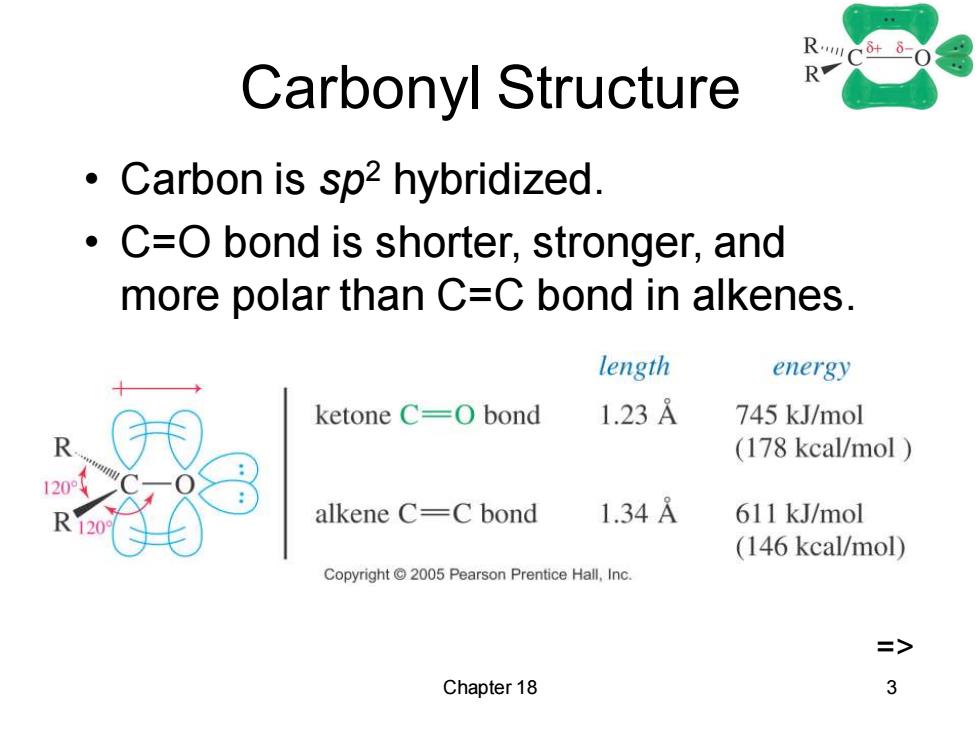
Carbonyl Structure R Carbon is sp2 hybridized. C=O bond is shorter,stronger,and more polar than C=C bond in alkenes. length energy ketone C=O bond 1.23A 745 kJ/mol (178 kcal/mol alkene C=C bond 1.34A 611 kJ/mol (146 kcal/mol) Copyright2005 Pearson Prentice Hall,Inc. Chapter 18 3Chapter 18 3 Carbonyl Structure • Carbon is sp2 hybridized. • C=O bond is shorter, stronger, and more polar than C=C bond in alkenes. =>