正在加载图片...
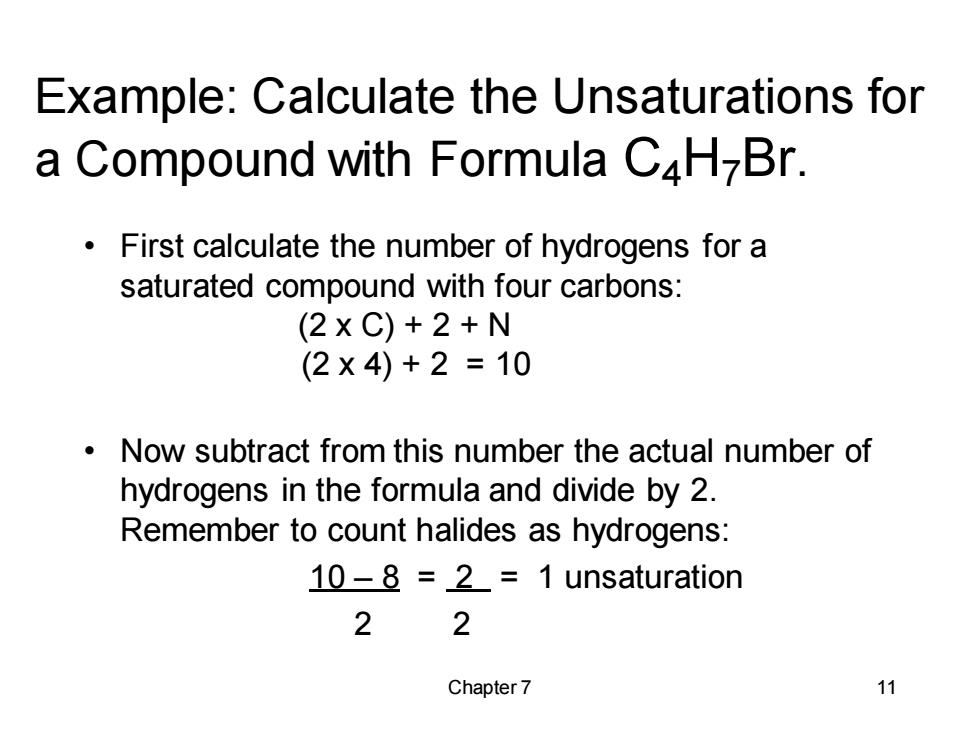
Example:Calculate the Unsaturations for a Compound with Formula C4H-Br. First calculate the number of hydrogens for a saturated compound with four carbons: (2xC)+2+N (2×4)+2=10 Now subtract from this number the actual number of hydrogens in the formula and divide by 2. Remember to count halides as hydrogens: 10-8 =2=1 unsaturation 2 2 Chapter 7 11Chapter 7 11 Example: Calculate the Unsaturations for a Compound with Formula C4H7Br. • First calculate the number of hydrogens for a saturated compound with four carbons: (2 x C) + 2 + N (2 x 4) + 2 = 10 • Now subtract from this number the actual number of hydrogens in the formula and divide by 2. Remember to count halides as hydrogens: 10 – 8 = 2 = 1 unsaturation 2 2