正在加载图片...
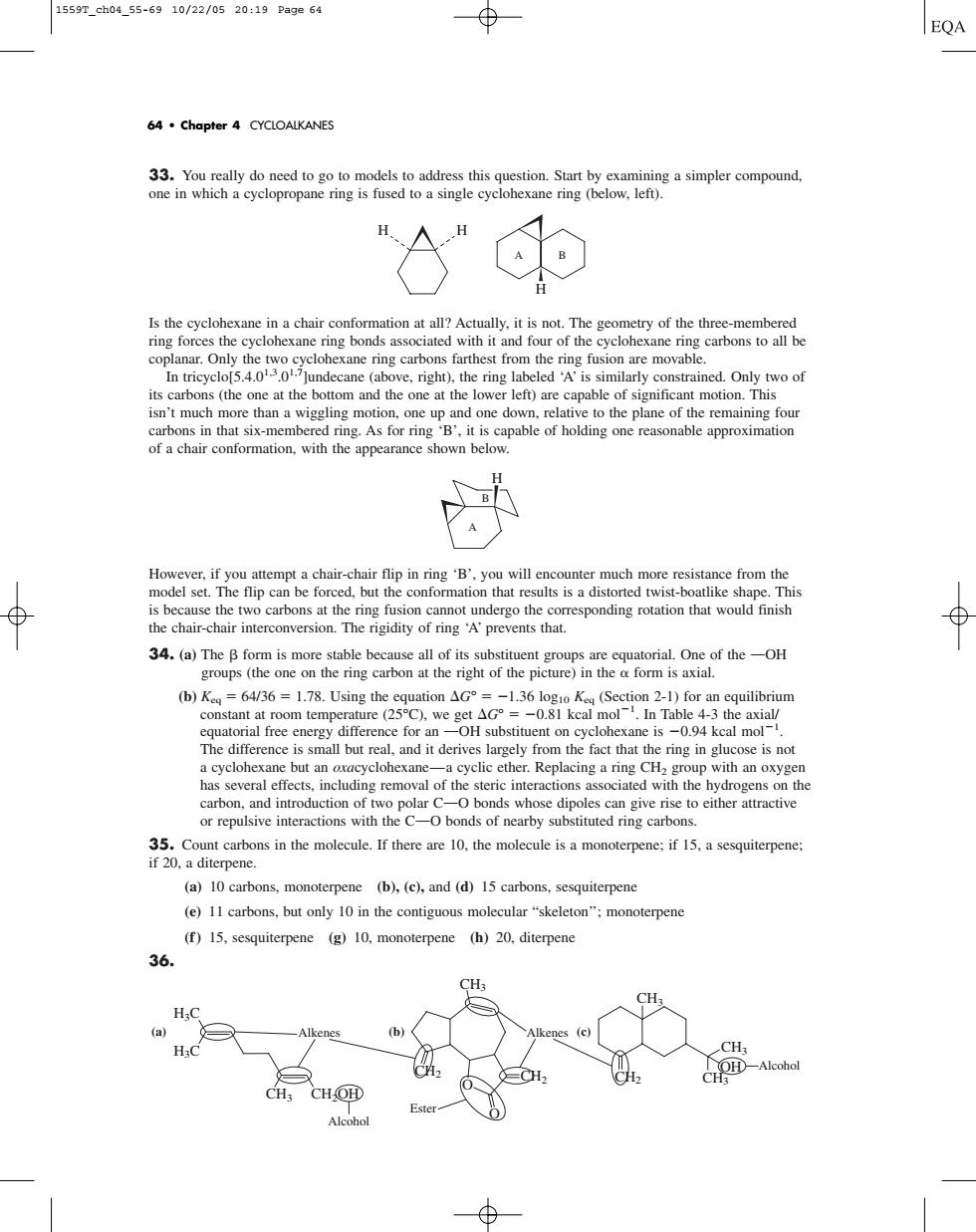
1559p.ch04_55-6910/22/0520:19Page64 64.Chapter 4 CYCLOALKANES left lohexane in a chair conformaion at all?Actually.it is not.The geometry of thethmem ring forces the c cyclohexane ring bonds asso ated with it and four of the of a chair conformation,with the appearance shown below. modeatempt a chair-chair flip ninByouwistoatke shape.This model se 3.e一o (DK=6436 17.the on( xane is -0.94 kcal mol- s largely from the fact thatt ring in glucose is no carbon,and introduction of two polar C-O bonds whose dipoles can give rise to either attractive or repulsive interactions with the CO bonds of nearby substituted ring carbon e mlleIhre ae 10.themol moerne. (a)10 carbons,monoterpene (b).(c).and (d)15 carbons,sesquiterpene (e)11 carbons.but only 10 in the contiguous molecular"skeleton":monoterpen (f)15.sesquiterpene (g)10.monoterpene (h)20.diterpene 36. CH. H;C (a) (b) CH. = CH Este33. You really do need to go to models to address this question. Start by examining a simpler compound, one in which a cyclopropane ring is fused to a single cyclohexane ring (below, left). Is the cyclohexane in a chair conformation at all? Actually, it is not. The geometry of the three-membered ring forces the cyclohexane ring bonds associated with it and four of the cyclohexane ring carbons to all be coplanar. Only the two cyclohexane ring carbons farthest from the ring fusion are movable. In tricyclo[5.4.01,3.01,7]undecane (above, right), the ring labeled ‘A’ is similarly constrained. Only two of its carbons (the one at the bottom and the one at the lower left) are capable of significant motion. This isn’t much more than a wiggling motion, one up and one down, relative to the plane of the remaining four carbons in that six-membered ring. As for ring ‘B’, it is capable of holding one reasonable approximation of a chair conformation, with the appearance shown below. However, if you attempt a chair-chair flip in ring ‘B’, you will encounter much more resistance from the model set. The flip can be forced, but the conformation that results is a distorted twist-boatlike shape. This is because the two carbons at the ring fusion cannot undergo the corresponding rotation that would finish the chair-chair interconversion. The rigidity of ring ‘A’ prevents that. 34. (a) The form is more stable because all of its substituent groups are equatorial. One of the OOH groups (the one on the ring carbon at the right of the picture) in the form is axial. (b) Keq 64/36 1.78. Using the equation G° 1.36 log10 Keq (Section 2-1) for an equilibrium constant at room temperature (25°C), we get G° 0.81 kcal mol1 . In Table 4-3 the axial/ equatorial free energy difference for an OOH substituent on cyclohexane is 0.94 kcal mol1 . The difference is small but real, and it derives largely from the fact that the ring in glucose is not a cyclohexane but an oxacyclohexane—a cyclic ether. Replacing a ring CH2 group with an oxygen has several effects, including removal of the steric interactions associated with the hydrogens on the carbon, and introduction of two polar COO bonds whose dipoles can give rise to either attractive or repulsive interactions with the COO bonds of nearby substituted ring carbons. 35. Count carbons in the molecule. If there are 10, the molecule is a monoterpene; if 15, a sesquiterpene; if 20, a diterpene. (a) 10 carbons, monoterpene (b), (c), and (d) 15 carbons, sesquiterpene (e) 11 carbons, but only 10 in the contiguous molecular “skeleton’’; monoterpene (f ) 15, sesquiterpene (g) 10, monoterpene (h) 20, diterpene 36. O O CH2 CH2 CH2 H3C H3C CH3 CH3 CH2OH CH3 OH CH3 CH3 Alkenes Alkenes Alcohol Ester (a) (b) (c) Alcohol H B A H A B H H 64 • Chapter 4 CYCLOALKANES 1559T_ch04_55-69 10/22/05 20:19 Page 64����