正在加载图片...
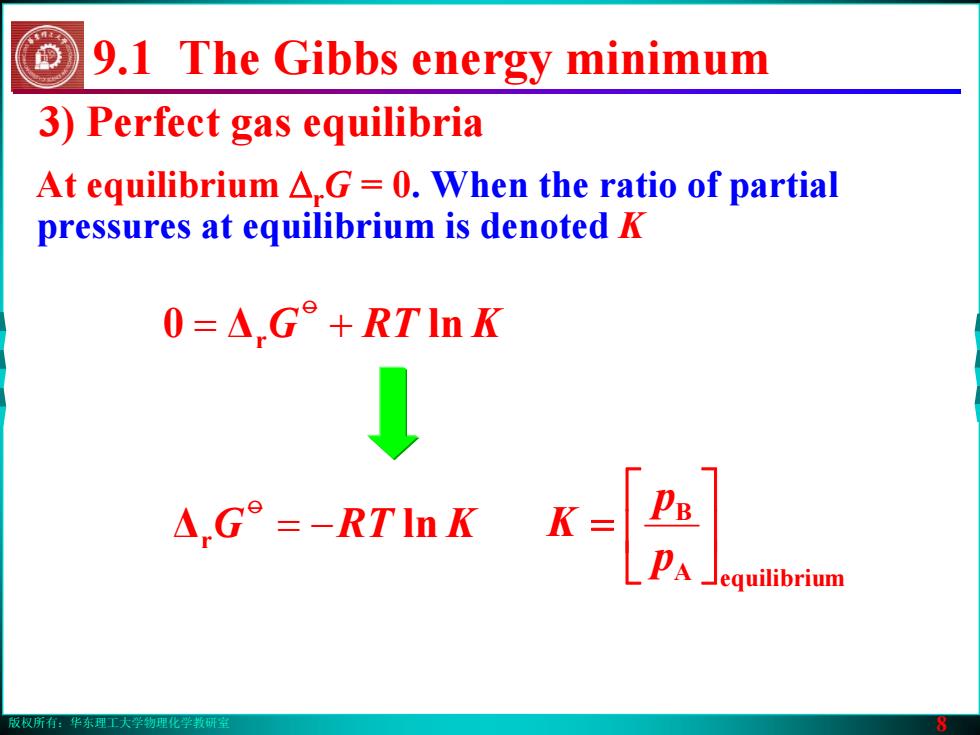
版权所有:华东理工大学物理化学教研室 8 3) Perfect gas equilibria At equilibrium ΔrG = 0. When the ratio of partial pressures at equilibrium is denoted K 9.1 The Gibbs energy minimum Δr −= ln KRTGo 0 Δr += ln KRTGo A equilibrium B ⎥⎦⎤ ⎢⎣⎡ = pp K版权所有:华东理工大学物理化学教研室 8 3) Perfect gas equilibria At equilibrium ΔrG = 0. When the ratio of partial pressures at equilibrium is denoted K 9.1 The Gibbs energy minimum Δr −= ln KRTGo 0 Δr += ln KRTGo A equilibrium B ⎥⎦⎤ ⎢⎣⎡ = pp K