正在加载图片...
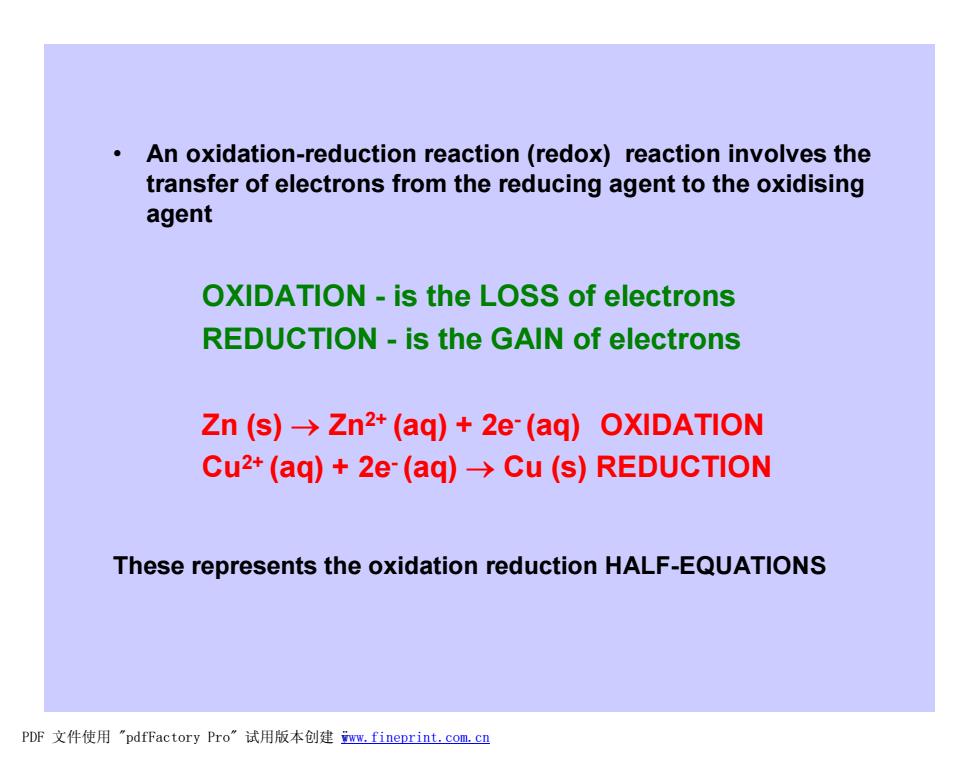
An oxidation-reduction reaction (redox)reaction involves the transfer of electrons from the reducing agent to the oxidising agent OXIDATION-is the LOSS of electrons REDUCTION-is the GAIN of electrons Zn (s)->Zn2+(aq)+2e-(aq)OXIDATION Cu2+(aq)+2e-(aq)->Cu (s)REDUCTION These represents the oxidation reduction HALF-EQUATIONS PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,c里 • An oxidation-reduction reaction (redox) reaction involves the transfer of electrons from the reducing agent to the oxidising agent OXIDATION - is the LOSS of electrons REDUCTION - is the GAIN of electrons Zn (s) ® Zn2+ (aq) + 2e- (aq) OXIDATION Cu2+ (aq) + 2e- (aq) ® Cu (s) REDUCTION These represents the oxidation reduction HALF-EQUATIONS PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn