正在加载图片...
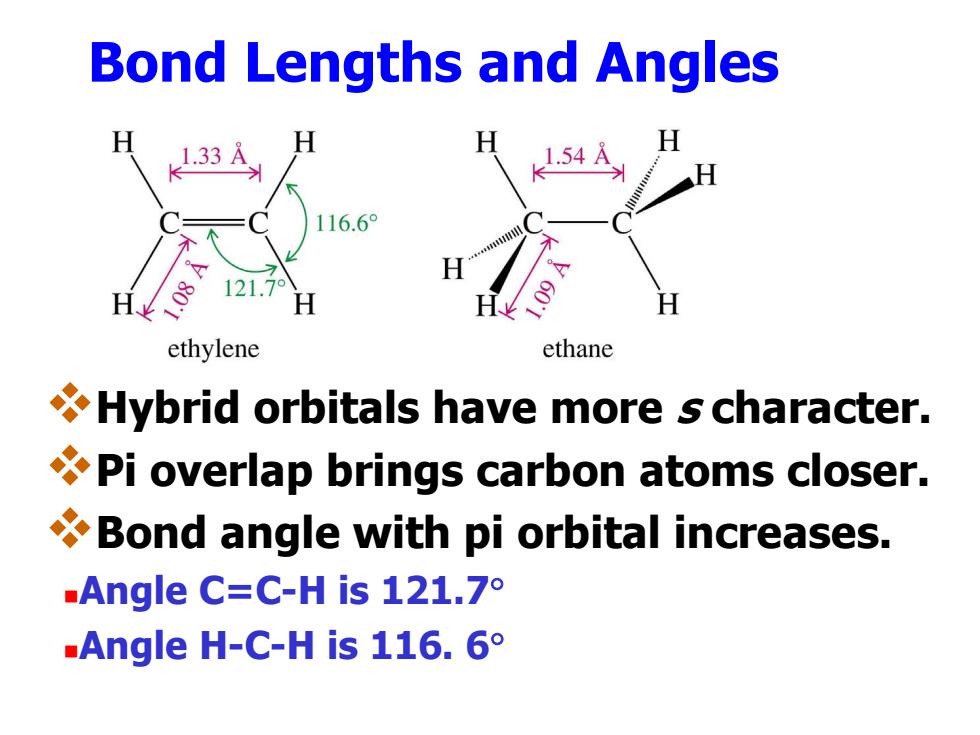
Bond Lengths and Angles 1.33 116.6° 121.7° ethylene ethane Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. .Angle C=C-His121.7° Angle H-C-His116.6° Bond Lengths and Angles Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. Angle C=C-H is 121.7 Angle H-C-H is 116. 6