正在加载图片...
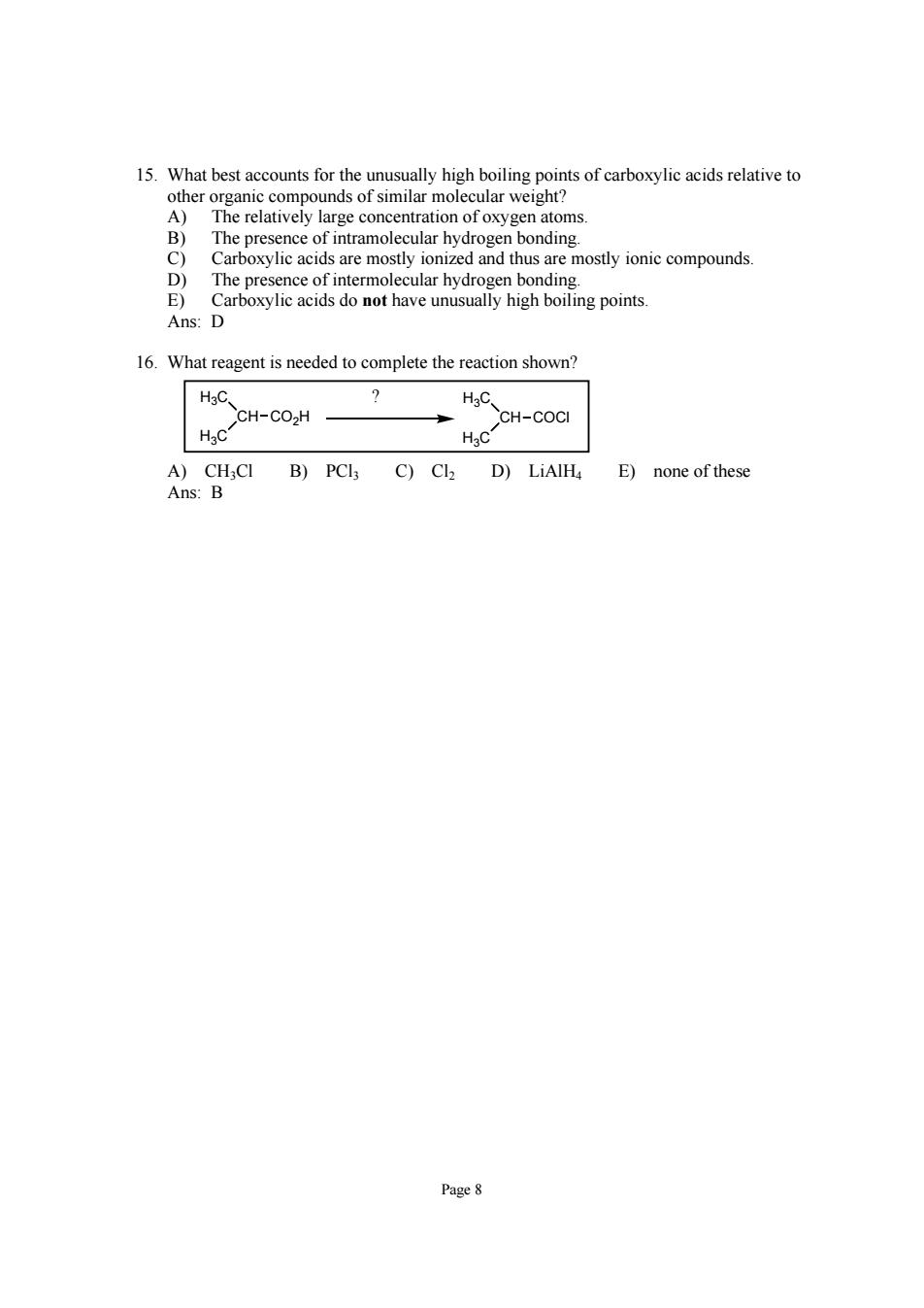
15.What bestacou for the high boiling points of i atoms The n bonding Cre mosothusr moty o cmound The presence of intermolecular hydrogen bonding. E)Carboxylic acids do not have unusually high boiling points. Ans:D 16.What reagent is needed to complete the reaction shown? CH-CO.H HC CH-COct A)CH,CI B)PCl3 C)Cl2 D)LiAlHa E)none of these Ans:B Page8 Page 8 15. What best accounts for the unusually high boiling points of carboxylic acids relative to other organic compounds of similar molecular weight? A) The relatively large concentration of oxygen atoms. B) The presence of intramolecular hydrogen bonding. C) Carboxylic acids are mostly ionized and thus are mostly ionic compounds. D) The presence of intermolecular hydrogen bonding. E) Carboxylic acids do not have unusually high boiling points. Ans: D 16. What reagent is needed to complete the reaction shown? H3C CH H3C CO2H H3C CH H3C COCl ? A) CH3Cl B) PCl3 C) Cl2 D) LiAlH4 E) none of these Ans: B