正在加载图片...
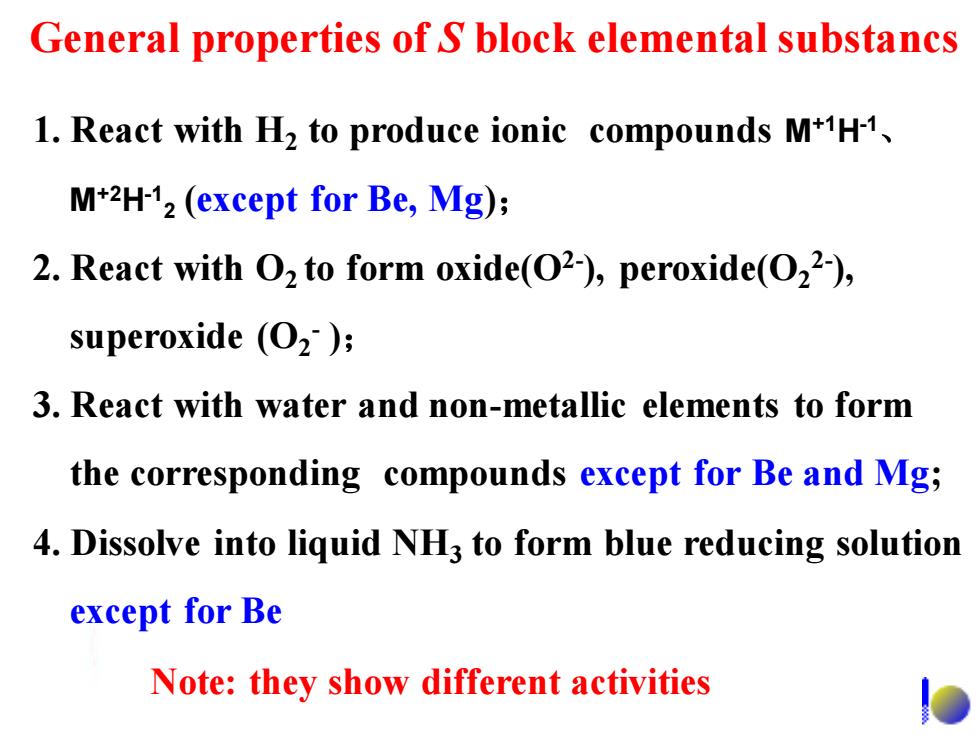
General properties of S block elemental substancs 1.React with H2 to produce ionic compounds M+H1, M+2H-12(except for Be,Mg); 2.React with O2 to form oxide(O2),peroxide(O22-), superoxide (O2); 3.React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4.Dissolve into liquid NH3 to form blue reducing solution except for Be Note:they show different activities1. React with H2 to produce ionic compounds M+1H-1 、 M+2H-1 2 (except for Be, Mg); 2. React with O2 to form oxide(O2- ), peroxide(O2 2- ), superoxide (O2 - ); 3. React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4. Dissolve into liquid NH3 to form blue reducing solution except for Be General properties of S block elemental substancs Note: they show different activities