正在加载图片...
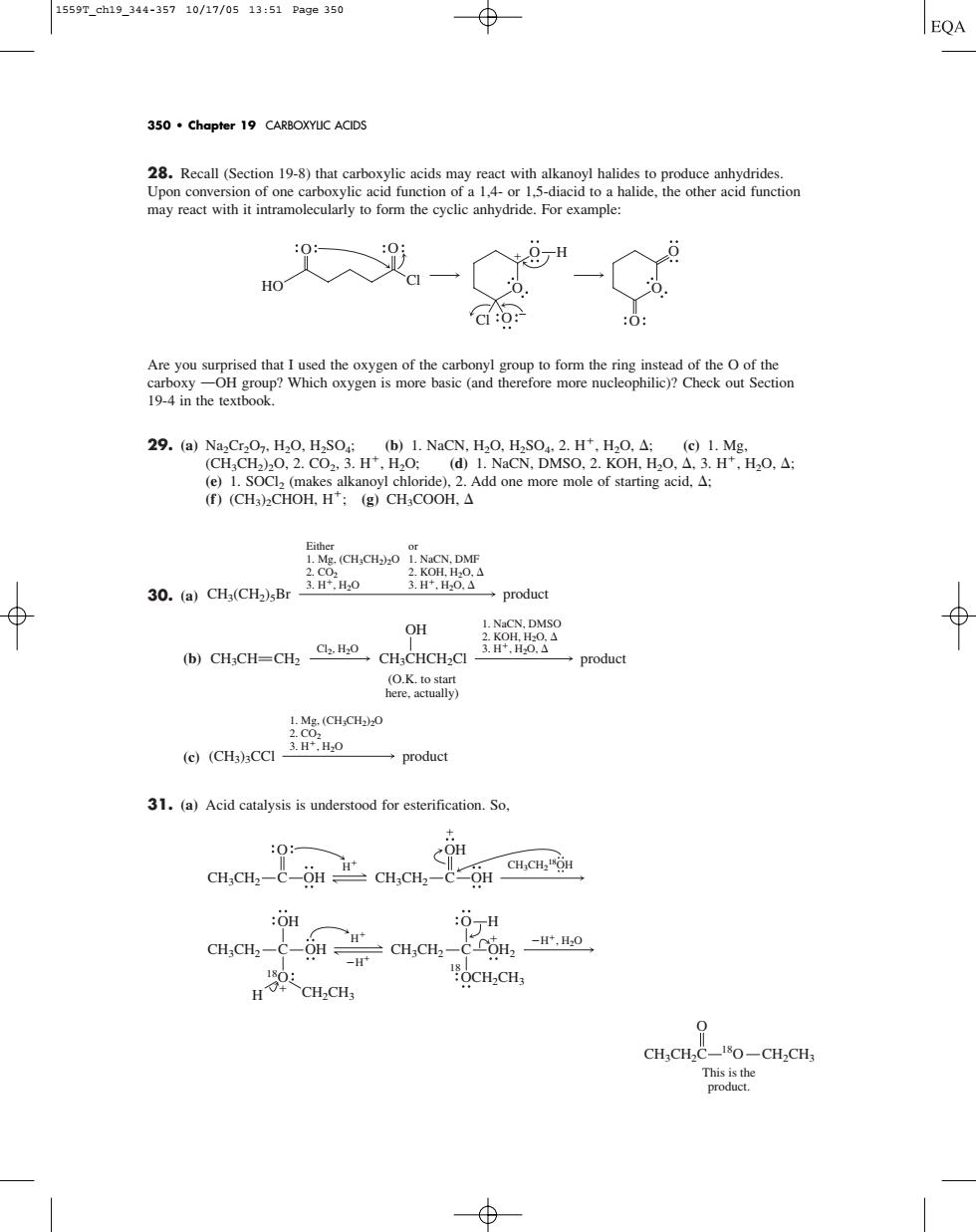
1559r.eh19.344-35710/17/0513:51Page350 350.chapter 19 CARBOXYUC ACIDS may reactwith it intramolecuyto form the cyclicahydride.For xampe: 0 H 6. -a (e)1.SOC(makes alkanoyl chloride).2.Add one more mole of starting acid.: (f)(CH3)2CHOH,H';(g)CHsCOOH.A 30.(a)CH:(CH2)sBr 盛器 →product OH bCH影CH=CH2aH -CH CHCHCI product y) 1.Mg.(CH.CH2)O product 31.(a)Acid catalysis is understood for esterification.So. CH.CH-e CH.CH GG :OH 6m,-o HCH.CH rche28. Recall (Section 19-8) that carboxylic acids may react with alkanoyl halides to produce anhydrides. Upon conversion of one carboxylic acid function of a 1,4- or 1,5-diacid to a halide, the other acid function may react with it intramolecularly to form the cyclic anhydride. For example: Are you surprised that I used the oxygen of the carbonyl group to form the ring instead of the O of the carboxy OOH group? Which oxygen is more basic (and therefore more nucleophilic)? Check out Section 19-4 in the textbook. 29. (a) Na2Cr2O7, H2O, H2SO4; (b) 1. NaCN, H2O, H2SO4, 2. H, H2O, ; (c) 1. Mg, (CH3CH2)2O, 2. CO2, 3. H, H2O; (d) 1. NaCN, DMSO, 2. KOH, H2O, , 3. H, H2O, ; (e) 1. SOCl2 (makes alkanoyl chloride), 2. Add one more mole of starting acid, ; (f) (CH3)2CHOH, H; (g) CH3COOH, 30. (a) (b) (c) 31. (a) Acid catalysis is understood for esterification. So, O This is the product. CH3CH2C 18O CH2CH3 18 OCH2CH3 CH3CH2 C OH H CH2CH3 OH 18O H H CH3CH2 C OH2 O H H, H2O CH3CH2 C OH O CH3CH2 C OH OH H CH3CH2 18OH 1. Mg, (CH3CH2)2O 2. CO2 3. H, H2O (CH3)3CCl product 1. NaCN, DMSO 2. KOH, H2O, 3. H Cl2, H2O , H2O, CH3CH CH2 CH3CHCH2Cl product OH (O.K. to start here, actually) Either 1. Mg, (CH3CH2)2O 2. CO2 3. H, H2O or 1. NaCN, DMF 2. KOH, H2O, 3. H, H2O, CH3(CH2)5Br product O O O O O O H Cl Cl O O HO 350 • Chapter 19 CARBOXYLIC ACIDS 1559T_ch19_344-357 10/17/05 13:51 Page 350����������������