正在加载图片...
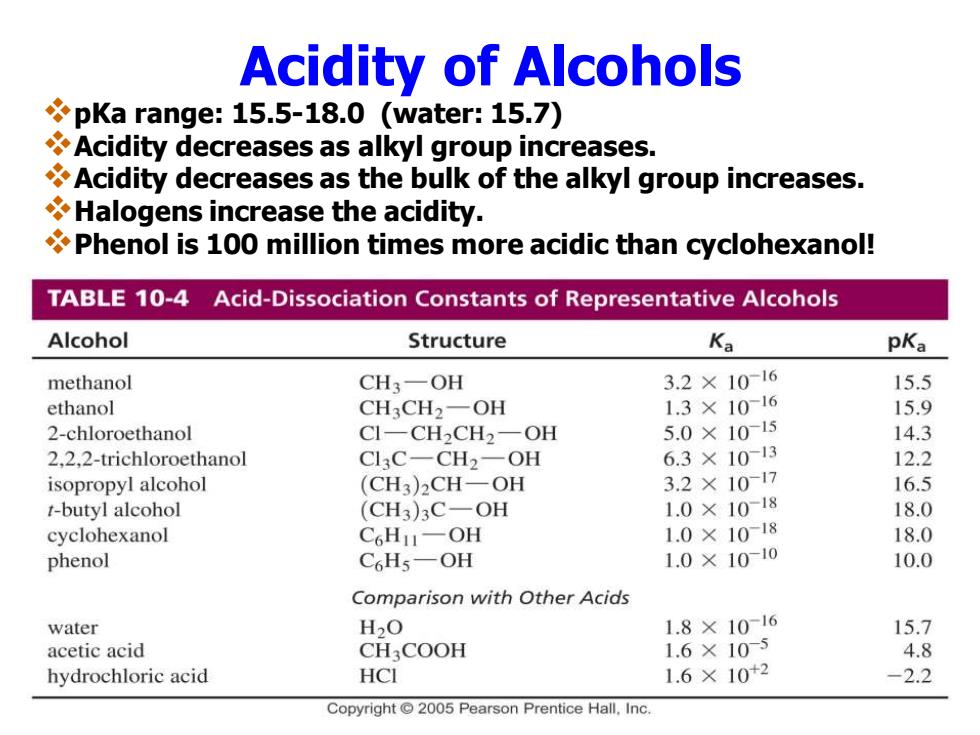
Acidity of Alcohols pKa range:15.5-18.0 (water:15.7) Acidity decreases as alkyl group increases. Acidity decreases as the bulk of the alkyl group increases. Halogens increase the acidity. Phenol is 100 million times more acidic than cyclohexanol! TABLE 10-4 Acid-Dissociation Constants of Representative Alcohols Alcohol Structure Ka pKa methanol CH3-OH 3.2×10-16 15.5 ethanol CH3CH2-OH 1.3×1016 15.9 2-chloroethanol CI-CH2CH2-OH 5.0×1015 14.3 2.2.2-trichloroethanol Cl3C-CH2-OH 6.3×1013 12.2 isopropyl alcohol (CH3)2CH-OH 3.2×1017 16.5 t-butyl alcohol (CH3)3C-OH 1.0×10-18 18.0 cyclohexanol C6HI-OH 1.0×1018 18.0 phenol C6Hs-OH 1.0×1010 10.0 Comparison with Other Acids water H2O 1.8×10-16 15.7 acetic acid CH3COOH 1.6×105 4.8 hydrochloric acid HCI 1.6×10+2 -2.2 Copyright 2005 Pearson Prentice Hall.Inc.Acidity of Alcohols ❖pKa range: 15.5-18.0 (water: 15.7) ❖Acidity decreases as alkyl group increases. ❖Acidity decreases as the bulk of the alkyl group increases. ❖Halogens increase the acidity. ❖Phenol is 100 million times more acidic than cyclohexanol!