正在加载图片...
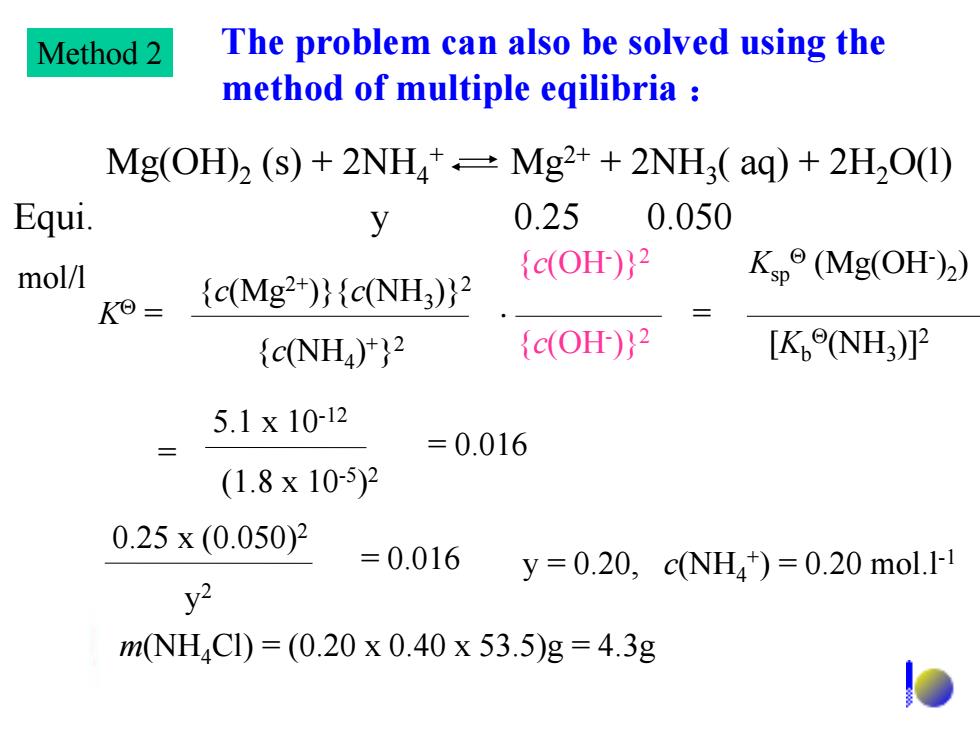
Method 2 The problem can also be solved using the method of multiple eqilibria Mg(OH)2 (s)+2NH*=Mg2+2NH3(aq)+2H2O(I) Equi. y 0.250.050 mol/l {c(OH)}2 Kp°(Mg(OHr)2) K0= {c(Mg2+)}{cNH3)}2 {c(NH4)}2 {c(OH)}2 [K,eNH3)]2 5.1x10-12 =0.016 (1.8x10-5)2 0.25x(0.050)2 =0.016 y=0.20,cNH4)=0.20mol.l y2 mNH4Cl)=(0.20x0.40x53.5)g=4.3g The problem can also be solved using the method of multiple eqilibria : Mg(OH)2 (s) + 2NH4 + Mg2+ + 2NH3 ( aq) + 2H2O(l) Equi. y 0.25 0.050 mol/l K = {c(Mg2+)}{c(NH3 )}2 {c(OH- )}2 {c(NH4 ) +} 2 {c(OH- )}2 = [Kb (NH3 )]2 Ksp (Mg(OH- )2 ) = 5.1 x 10-12 (1.8 x 10-5 ) 2 = 0.016 0.25 x (0.050)2 y 2 = 0.016 y = 0.20, c(NH4 + ) = 0.20 mol.l-1 m(NH4Cl) = (0.20 x 0.40 x 53.5)g = 4.3g Method 2