正在加载图片...
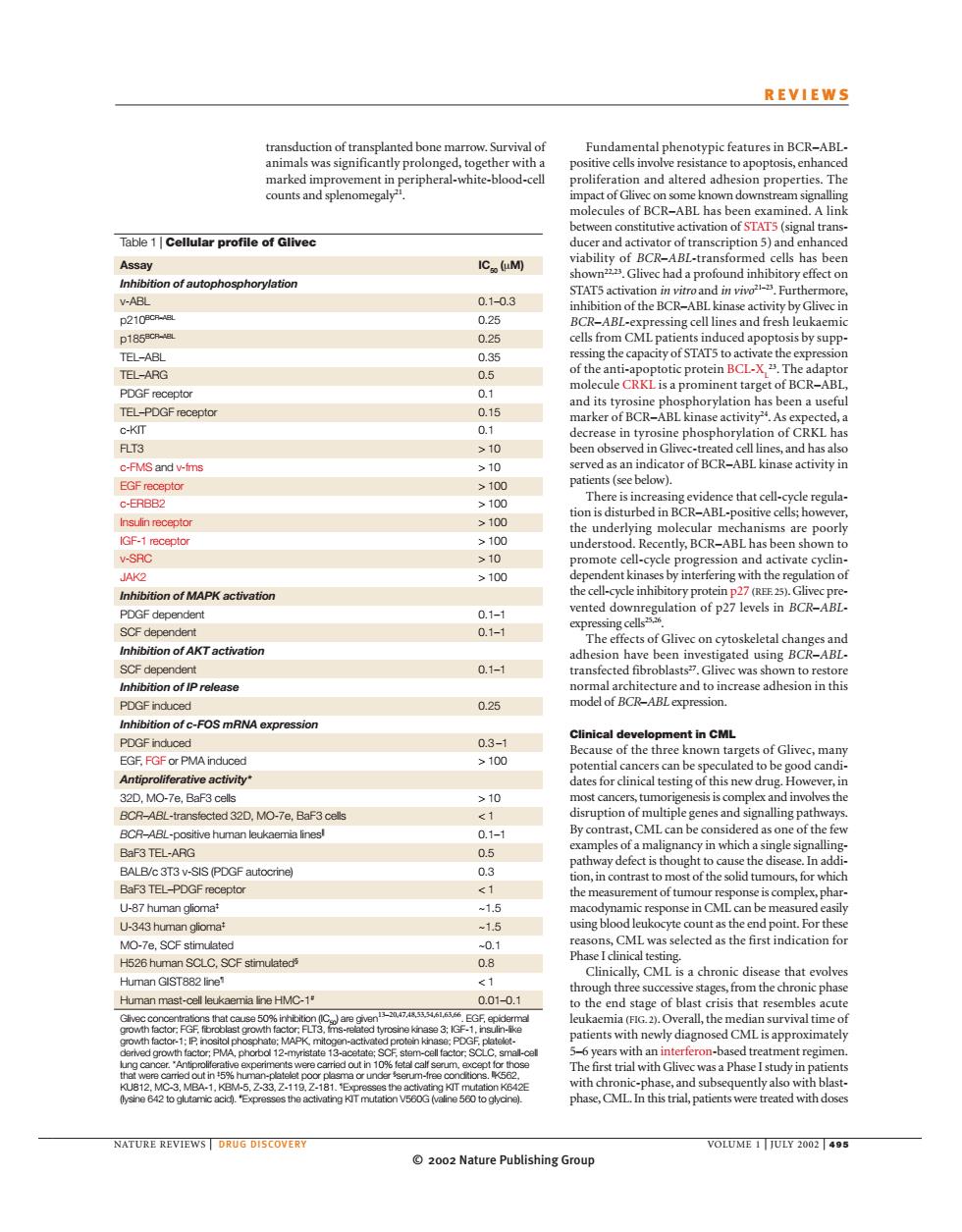
REVIEWS ransduction of transplanted bone marrow.Survival of Fundamental phenotypic features in BCR-ABL ty pro link Table 1|Cellular nconstitutieaciationofS7 A长 IC(M) V-AB 0.1-0.3 STAT5 activa 210 月A g of the TEL-ARG 0.5 ttarget of BCR- DGF recepto DGF recepte arker ofBCR-ABL kina d. C-KT FLT3 10 id ha c-FMS and w-ims >10 2100 ≥100 IGF-1epo understood.Recently.BCR-ABL has ent kin lation o Inhibition of MAPK activation ented downre PDGF dependen 0.1- Gliv n of AKT activation 0.1-4 to re 0.26 nof-FOS mRNA expression 0.3-1 >100 dcnan lates for cli▣ al testing o BCR-ABL-transtected 32D.MO-7e.BaF3 cels genes anc BCR-ABL-postive human loukaemia linesl 0.1- BaF3 TEL-PDGF receptor ontrast t U-87 human glioma -15 -343om CML was H526 human SCLC,SCF stimulated 0.8 e that evolve Human GIST882 line <1 om p. Human mast-cel leuka 0.01-0 f bl sis th atients with diagno is approxin 1, RE REVIEWS DRUG DISCOVER OLUME 200 2002 Nature Publishing Group© 2002 Nature Publishing Group NATURE REVIEWS | DRUG DISCOVERY VOLUME 1 | JULY 2002 | 495 REVIEWS Fundamental phenotypic features in BCR–ABLpositive cells involve resistance to apoptosis, enhanced proliferation and altered adhesion properties. The impact of Glivec on some known downstream signalling molecules of BCR–ABL has been examined. A link between constitutive activation of STAT5 (signal transducer and activator of transcription 5) and enhanced viability of BCR–ABL-transformed cells has been shown22,23. Glivec had a profound inhibitory effect on STAT5 activation in vitro and in vivo21–23. Furthermore, inhibition of the BCR–ABL kinase activity by Glivec in BCR–ABL-expressing cell lines and fresh leukaemic cells from CML patients induced apoptosis by suppressing the capacity of STAT5 to activate the expression of the anti-apoptotic protein BCL-XL 23. The adaptor molecule CRKL is a prominent target of BCR–ABL, and its tyrosine phosphorylation has been a useful marker of BCR–ABL kinase activity24. As expected, a decrease in tyrosine phosphorylation of CRKL has been observed in Glivec-treated cell lines, and has also served as an indicator of BCR–ABL kinase activity in patients (see below). There is increasing evidence that cell-cycle regulation is disturbed in BCR–ABL-positive cells; however, the underlying molecular mechanisms are poorly understood. Recently, BCR–ABL has been shown to promote cell-cycle progression and activate cyclindependent kinases by interfering with the regulation of the cell-cycle inhibitory protein p27 (REF. 25). Glivec prevented downregulation of p27 levels in BCR–ABLexpressing cells25,26. The effects of Glivec on cytoskeletal changes and adhesion have been investigated using BCR–ABLtransfected fibroblasts27. Glivec was shown to restore normal architecture and to increase adhesion in this model of BCR–ABL expression. Clinical development in CML Because of the three known targets of Glivec, many potential cancers can be speculated to be good candidates for clinical testing of this new drug. However, in most cancers, tumorigenesis is complex and involves the disruption of multiple genes and signalling pathways. By contrast, CML can be considered as one of the few examples of a malignancy in which a single signallingpathway defect is thought to cause the disease. In addition, in contrast to most of the solid tumours, for which the measurement of tumour response is complex, pharmacodynamic response in CML can be measured easily using blood leukocyte count as the end point. For these reasons, CML was selected as the first indication for Phase I clinical testing. Clinically, CML is a chronic disease that evolves through three successive stages, from the chronic phase to the end stage of blast crisis that resembles acute leukaemia (FIG. 2). Overall, the median survival time of patients with newly diagnosed CML is approximately 5–6 years with an interferon-based treatment regimen. The first trial with Glivec was a Phase I study in patients with chronic-phase, and subsequently also with blastphase, CML. In this trial, patients were treated with doses transduction of transplanted bone marrow. Survival of animals was significantly prolonged, together with a marked improvement in peripheral-white-blood-cell counts and splenomegaly21. Table 1 | Cellular profile of Glivec Assay IC50 (µM) Inhibition of autophosphorylation v-ABL 0.1–0.3 p210BCR–ABL 0.25 p185BCR–ABL 0.25 TEL–ABL 0.35 TEL–ARG 0.5 PDGF receptor 0.1 TEL–PDGF receptor 0.15 c-KIT 0.1 FLT3 > 10 c-FMS and v-fms > 10 EGF receptor > 100 c-ERBB2 > 100 Insulin receptor > 100 IGF-1 receptor > 100 v-SRC > 10 JAK2 > 100 Inhibition of MAPK activation PDGF dependent 0.1–1 SCF dependent 0.1–1 Inhibition of AKT activation SCF dependent 0.1–1 Inhibition of IP release PDGF induced 0.25 Inhibition of c-FOS mRNA expression PDGF induced 0.3 –1 EGF, FGF or PMA induced > 100 Antiproliferative activity* 32D, MO-7e, BaF3 cells > 10 BCR–ABL-transfected 32D, MO-7e, BaF3 cells < 1 BCR–ABL-positive human leukaemia lines|| 0.1–1 BaF3 TEL-ARG 0.5 BALB/c 3T3 v-SIS (PDGF autocrine) 0.3 BaF3 TEL–PDGF receptor < 1 U-87 human glioma‡ ~1.5 U-343 human glioma‡ ~1.5 MO-7e, SCF stimulated ~0.1 H526 human SCLC, SCF stimulated§ 0.8 Human GIST882 line¶ < 1 Human mast-cell leukaemia line HMC-1# 0.01–0.1 Glivec concentrations that cause 50% inhibition (IC50) are given13–20,47,48,53,54,61,63,66. EGF, epidermal growth factor; FGF, fibroblast growth factor; FLT3, fms-related tyrosine kinase 3; IGF-1, insulin-like growth factor-1; IP, inositol phosphate; MAPK, mitogen-activated protein kinase; PDGF, plateletderived growth factor; PMA, phorbol 12-myristate 13-acetate; SCF, stem-cell factor; SCLC, small-cell lung cancer. *Antiproliferative experiments were carried out in 10% fetal calf serum, except for those that were carried out in ‡ 5% human-platelet poor plasma or under § serum-free conditions. ||K562, KU812, MC-3, MBA-1, KBM-5, Z-33, Z-119, Z-181. ¶Expresses the activating KIT mutation K642E (lysine 642 to glutamic acid). # Expresses the activating KIT mutation V560G (valine 560 to glycine)