正在加载图片...
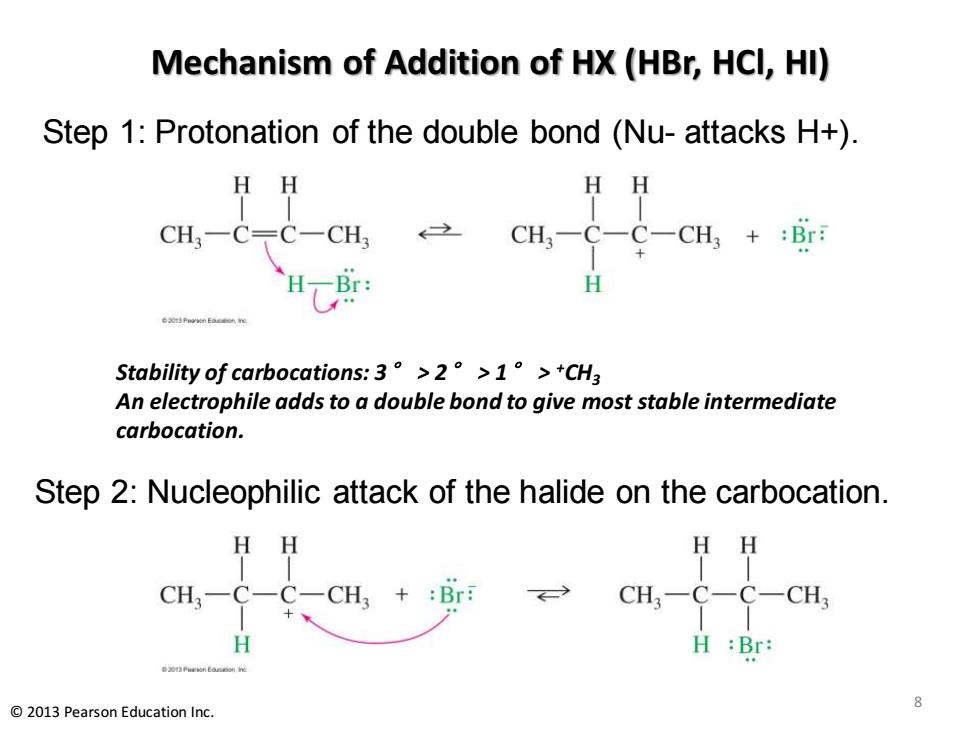
Mechanism of Addition of HX(HBr,HCl,HI) Step 1:Protonation of the double bond (Nu-attacks H+) HH HH CH3一C〒C-CH CH3-C-C-CH3+:Br: H Stability of carbocations:.3°>2°>1。>+CH3 An electrophile adds to a double bond to give most stable intermediate carbocation. Step 2:Nucleophilic attack of the halide on the carbocation. HH HH CH3一C-C一CH3+:Br CH3-C-C- CH H H :Br: 2013 Pearson Education Inc. Mechanism of Addition of HX (HBr, HCl, HI) Step 1: Protonation of the double bond (Nu- attacks H+). Step 2: Nucleophilic attack of the halide on the carbocation. 8 © 2013 Pearson Education Inc. Stability of carbocations: 3° > 2° > 1° > +CH3 An electrophile adds to a double bond to give most stable intermediate carbocation