正在加载图片...
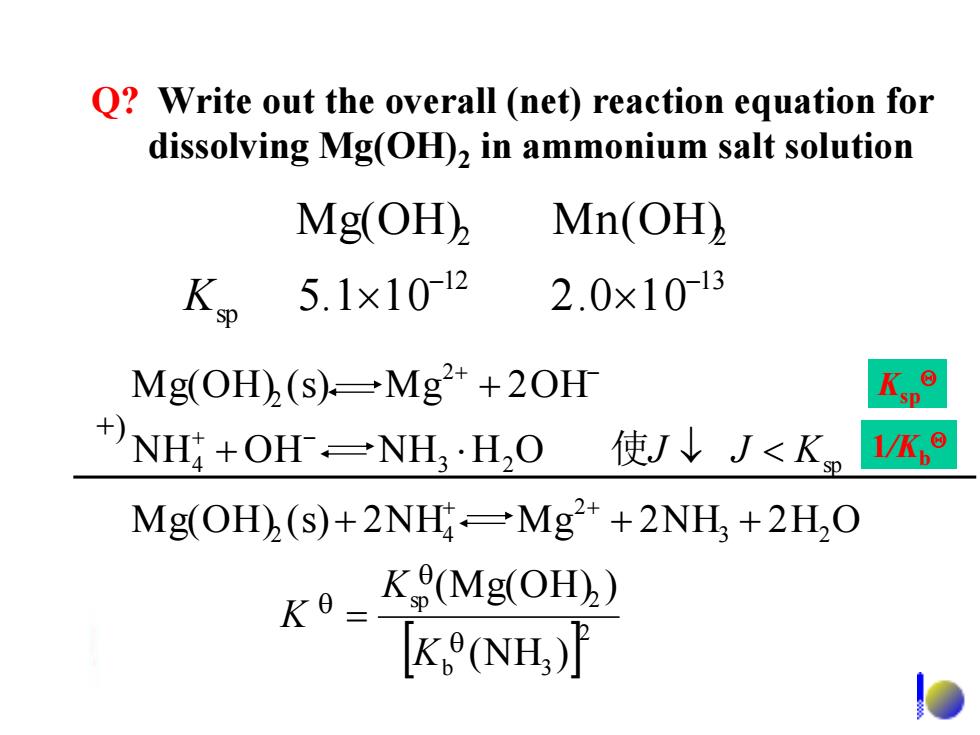
Q?Write out the overall (net)reaction equation for dissolving Mg(OH)2 in ammonium salt solution Mg(OH), Mn(OH) 5.1×1012 2.0x1013 Mg(OH)(s)-Mg+20H +)NH+OHr=NH·HO 使J↓J<K 1/K,e Mg(OH)(s)+2NH-Mg2*+2NH,+2H,O K K(Mg(OH)) K (NH)12 13 sp 2 2 5. 1 1 0 2.0 1 0 Mg(OH) Mn(OH) - - K 2 b 3 sp 2 3 2 2 2 4 4 3 2 sp 2 2 (NH ) (Mg(OH) ) Mg(OH) (s) 2NH M g 2NH 2 H O N H O H N H H O Mg(OH) (s) M g 2OH K K K J J K = + + + + < + + + + - + - 使 +) Ksp 1/Kb Q? Write out the overall (net) reaction equation for dissolving Mg(OH)2 in ammonium salt solution