正在加载图片...
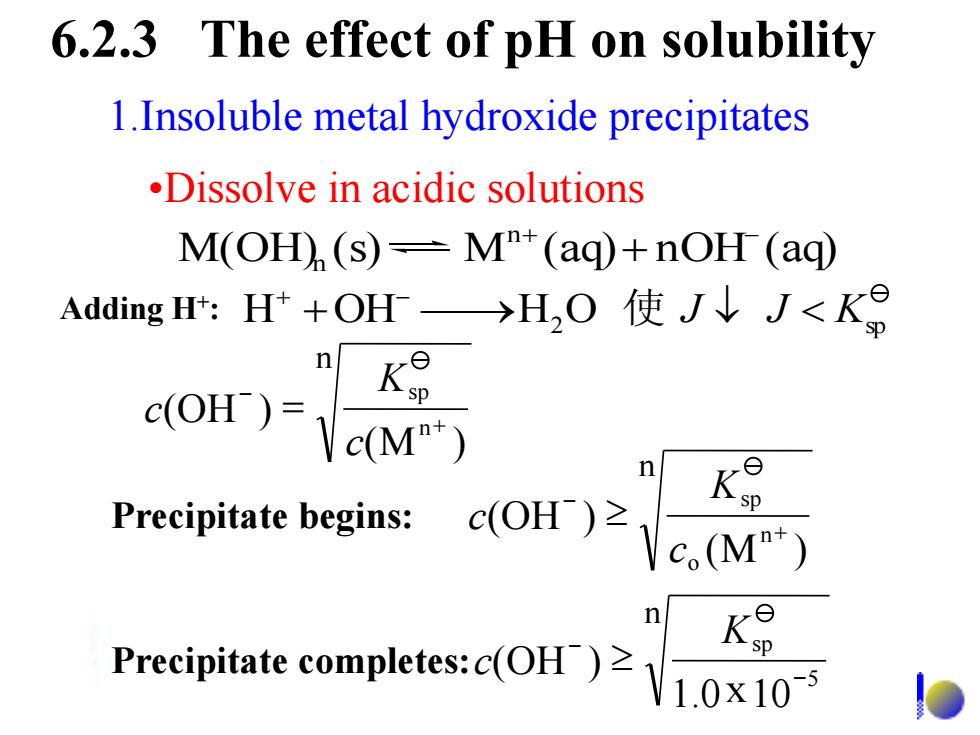
6.2.3 The effect of pH on solubility 1.Insoluble metal hydroxide precipitates .Dissolve in acidic solutions M(OH(s)=MT(aq)+nOH(aq) Adding H:H+OH→H2O使J↓J<Kg n K是 c(OH)= Vc(M") n K Precipitate begins:c(OH) c(M") K Precipitate completes:c(OH V1.0x1051.Insoluble metal hydroxide precipitates •Dissolve in acidic solutions M(OH) (s) M (aq) nOH (aq) n n + - + 6.2.3 The effect of pH on solubility n n sp (M ) (OH ) + - = c K c n 5 sp 1.0 10 (OH ) - - x K Precipitate completes: c n n o sp (M ) (OH ) + - c K Precipitate begins: c 2 sp H +OH H O J J < K Adding H 加酸, + : + - 使