正在加载图片...
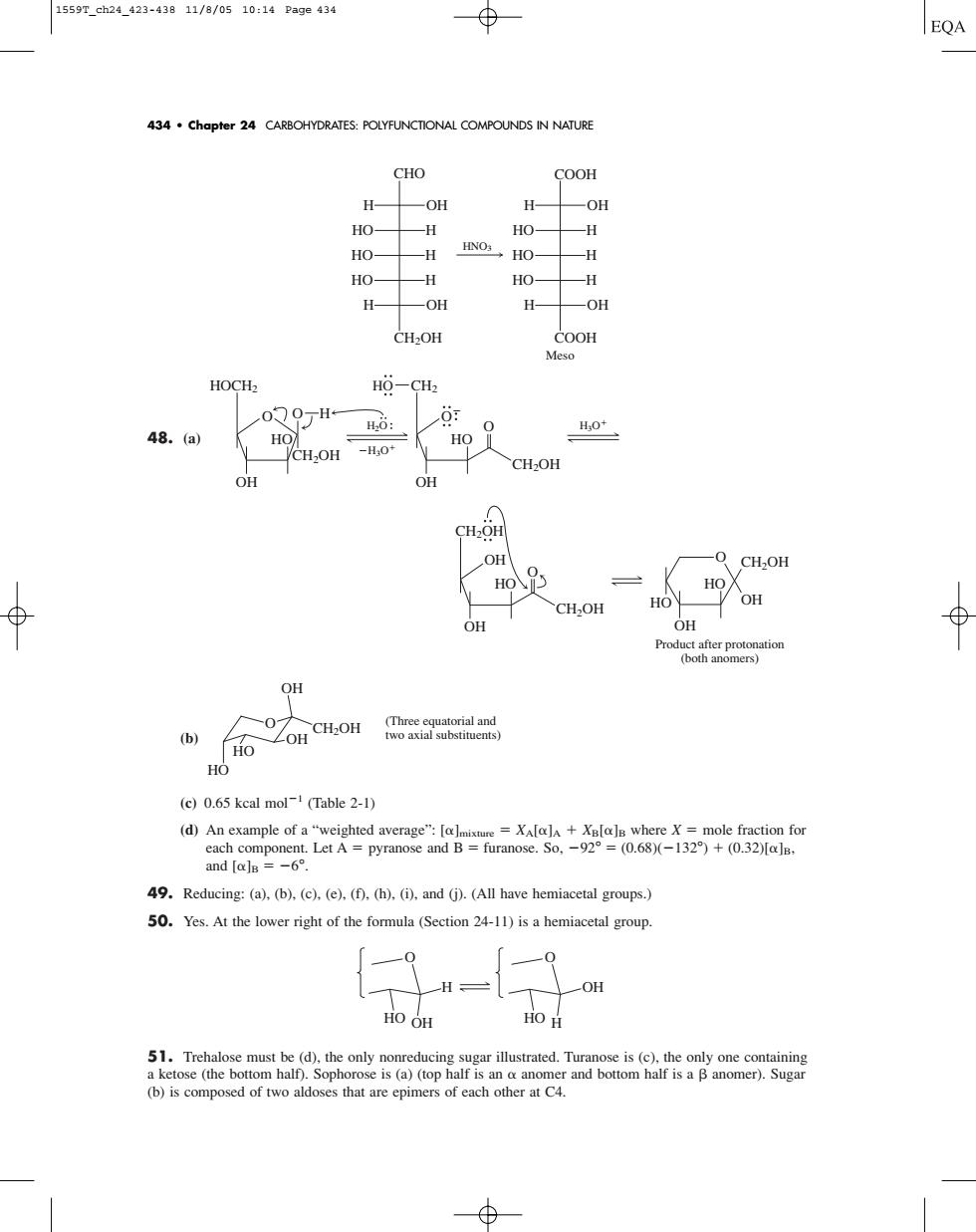
1559Tch24423-43811/8/0510:14Page434 434.Chapter 24 CARBOHYDRATES:POLYFUNCTIONAL COMPOUNDS IN NATURE CHO COOH H OH H- OH HO- -H HO- 一H HO 一H HNO: HO- -H HO- -H HO- -H H- OH H OH CH 20H Meso HOCH HO-CHz 0Q0万H 6: 0 H0* 48.(a HO HO -H:0 CH2OH H OH -0 CH2OH HO HO OH OH OH Phodetterprotogatiod Ho ZOH CH:OH oacmolng (c)0.65 kcal mol (Table 2-1) and[alg=-6° 49.Reducing:(a),(b).(c).(e).(f).(h).(i).and (j).(All have hemiacetal groups.) 50.Yes.At the lower right of the formula(Section 24-11)is a hemiacetal group HO OH 51.Trehalose must be (d),the only nonreducing sugar illustrated.Turanose is (c),the only one containing of two a48. (a) (b) (c) 0.65 kcal mol1 (Table 2-1) (d) An example of a “weighted average”: []mixture XA[]A XB[]B where X mole fraction for each component. Let A pyranose and B furanose. So, 92° (0.68)(132°) (0.32)[]B, and []B 6°. 49. Reducing: (a), (b), (c), (e), (f), (h), (i), and (j). (All have hemiacetal groups.) 50. Yes. At the lower right of the formula (Section 24-11) is a hemiacetal group. 51. Trehalose must be (d), the only nonreducing sugar illustrated. Turanose is (c), the only one containing a ketose (the bottom half). Sophorose is (a) (top half is an anomer and bottom half is a anomer). Sugar (b) is composed of two aldoses that are epimers of each other at C4. O HO H OH O HO OH H O HO CH2OH HO OH OH (Three equatorial and two axial substituents) OH CH2OH CH2OH HO OH O Product after protonation (both anomers) CH2OH HO HO OH OH O H2O H3O H3O HO H HOCH2 OH CH2OH HO O O CH2 OH CH2OH HO O O HNO3 COOH COOH Meso CH2OH CHO HO H HO H HO H H OH H OH H OH HO H HO H HO H H OH 434 • Chapter 24 CARBOHYDRATES: POLYFUNCTIONAL COMPOUNDS IN NATURE 1559T_ch24_423-438 11/8/05 10:14 Page 434�������