正在加载图片...
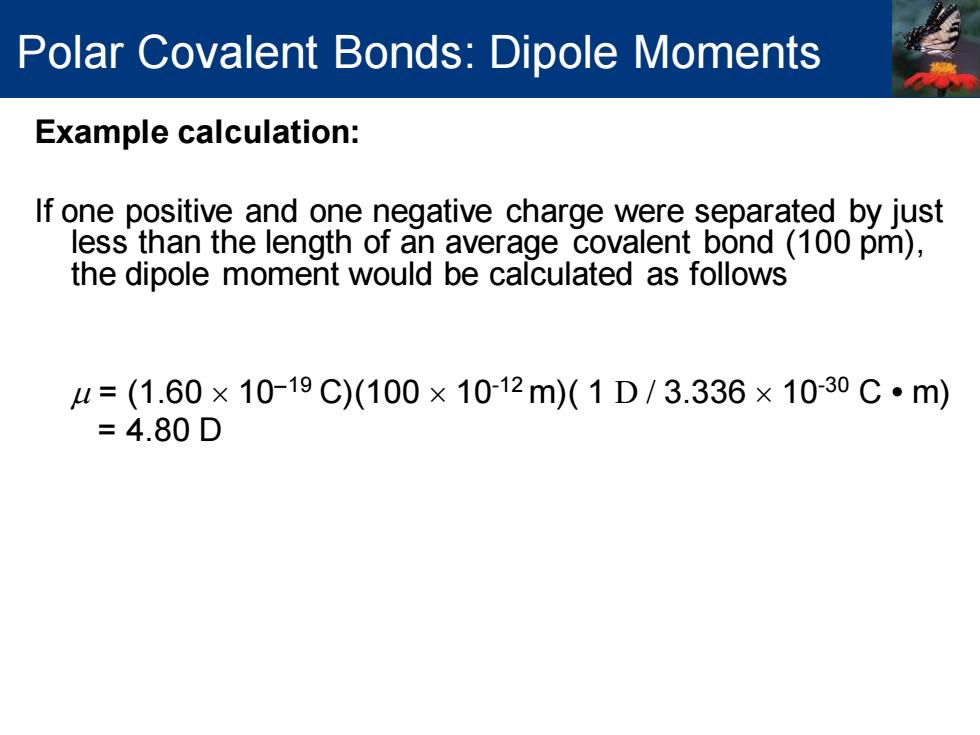
Polar Covalent Bonds:Dipole Moments Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows =(1.60×10-19C)(100×1012m)(1D/3.336×10-30C·m) =4.80D Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows = (1.60 10−19 C)(100 10-12 m)( 1 D / 3.336 10-30 C • m) = 4.80 D Polar Covalent Bonds: Dipole Moments