正在加载图片...
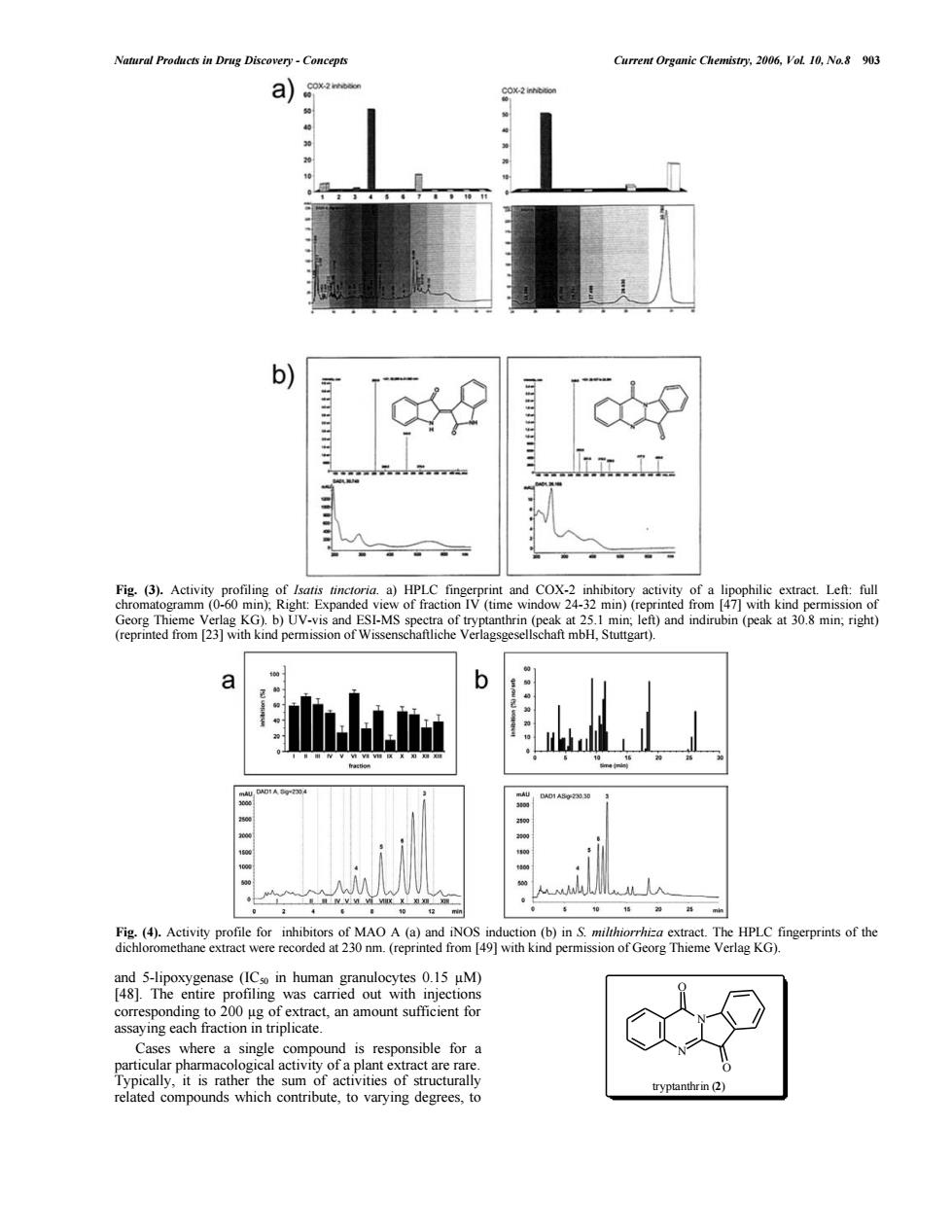
Natural Products in Drug Discovery-Concepts Current Organic Chemistry.2006.VoL 10,No.8 903 a) b) Fig.(3). a)HPLC COX-2 in ory acuvity rep haft m H,Stuttgart). (b)The HPLC fingerprints of the sion of Georg Thieme Verlag KG). d is particular pharma reomodsueto varyingerto tryptanthrin (2)Natural Products in Drug Discovery - Concepts Current Organic Chemistry, 2006, Vol. 10, No.8 903 and 5-lipoxygenase (IC50 in human granulocytes 0.15 µM) [48]. The entire profiling was carried out with injections corresponding to 200 µg of extract, an amount sufficient for assaying each fraction in triplicate. Cases where a single compound is responsible for a particular pharmacological activity of a plant extract are rare. Typically, it is rather the sum of activities of structurally related compounds which contribute, to varying degrees, to Fig. (3). Activity profiling of Isatis tinctoria. a) HPLC fingerprint and COX-2 inhibitory activity of a lipophilic extract. Left: full chromatogramm (0-60 min); Right: Expanded view of fraction IV (time window 24-32 min) (reprinted from [47] with kind permission of Georg Thieme Verlag KG). b) UV-vis and ESI-MS spectra of tryptanthrin (peak at 25.1 min; left) and indirubin (peak at 30.8 min; right) (reprinted from [23] with kind permission of Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart). Fig. (4). Activity profile for inhibitors of MAO A (a) and iNOS induction (b) in S. milthiorrhiza extract. The HPLC fingerprints of the dichloromethane extract were recorded at 230 nm. (reprinted from [49] with kind permission of Georg Thieme Verlag KG). N N O O tryptanthrin (2)