正在加载图片...
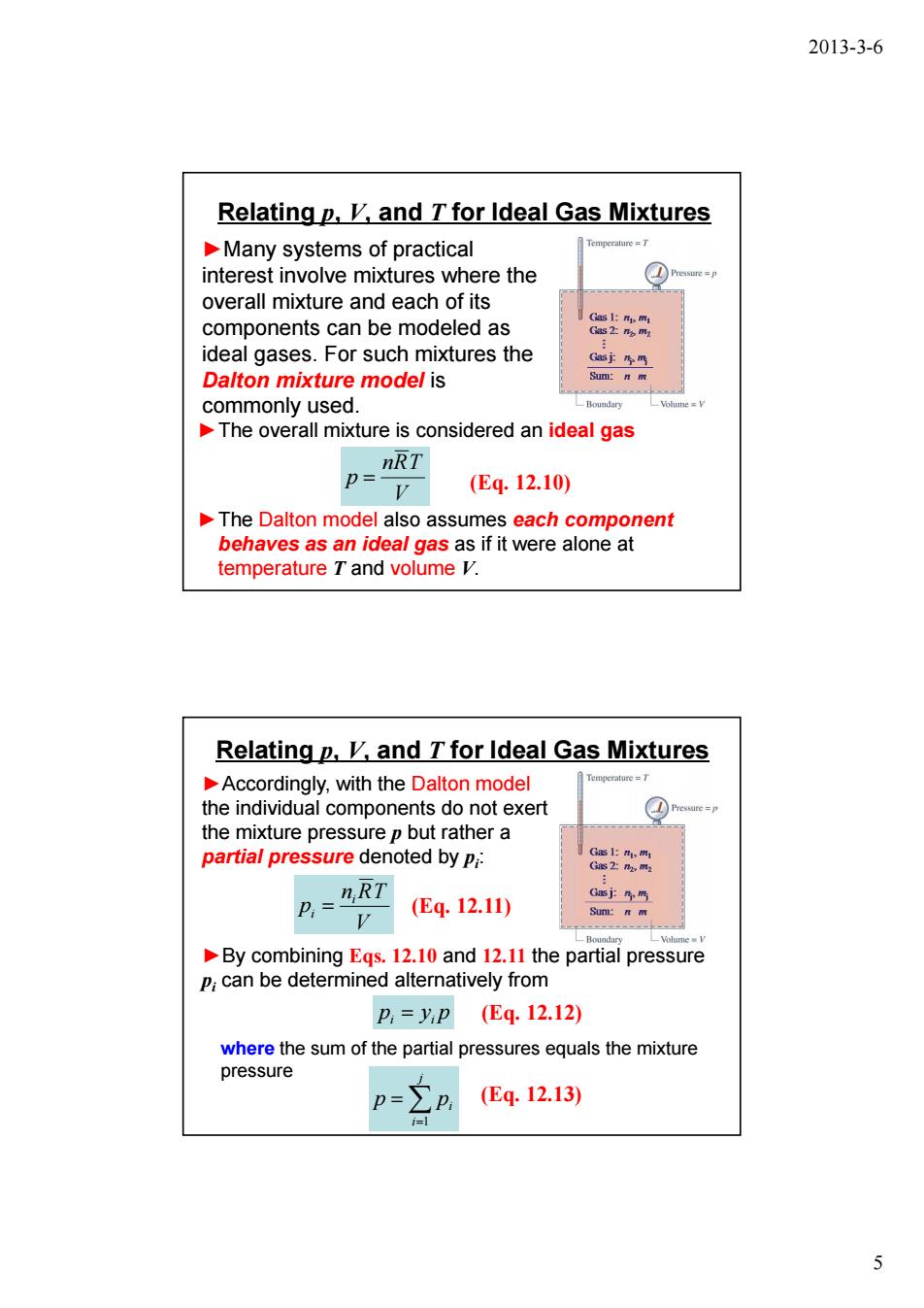
2013-3-6 Relatingp,K,and T for Ideal Gas Mixtures Many systems of practical interest involve mixtures where the overall mixture and each of its components can be modeled as ideal gases.For such mixtures the Dalton mixture model is commonly used. The overall mixture is considered an ideal gas P=IRT V (E4.12.10) The Dalton model also assumes each component behaves as an ideal gas as if it were alone at temperature T and volume V. Relating p,K,and T for Ideal Gas Mixtures Accordingly,with the Dalton model the individual components do not exert the mixture pressure p but rather a partial pressure denoted by Pi: (E4.12.11) V By combining Egs.12.10 and 12.11 the partial pressure P;can be determined alternatively from p,=yP(Eq.12.12) where the sum of the partial pressures equals the mixture pressure p=Ep (Eq.12.13) 5 2013-3-6 5 Relating p, V, and T for Ideal Gas Mixtures ►Many systems of practical interest involve mixtures where the overall mixture and each of its components can be modeled as ideal gases. For such mixtures the Dalton mixture model is commonly used. ►The overall mixture is considered an ideal gas V nRT p = (Eq. 12.10) ►The Dalton model also assumes each component behaves as an ideal gas as if it were alone at temperature T and volume V. Relating p, V, and T for Ideal Gas Mixtures ►Accordingly, with the Dalton model the individual components do not exert the mixture pressure p but rather a partial pressure denoted by pi : V n RT p i i = (Eq. 12.11) p y p i = i (Eq. 12.12) ►By combining Eqs. 12.10 and 12.11 the partial pressure pi can be determined alternatively from where the sum of the partial pressures equals the mixture pressure ∑ (Eq. 12.13) = = j i p pi 1