正在加载图片...
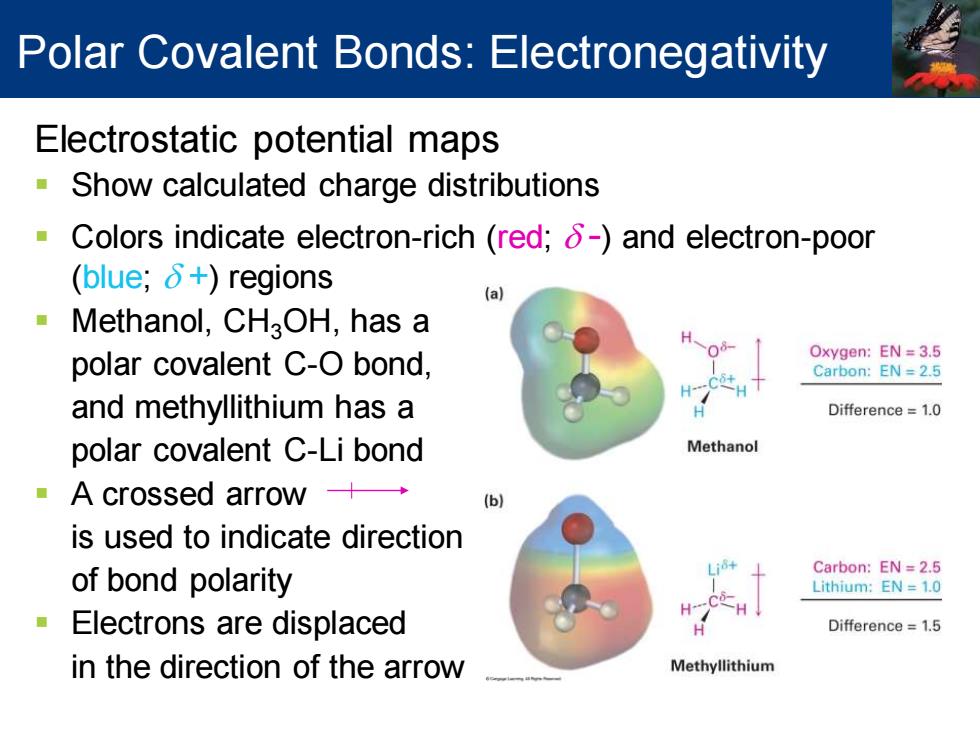
Polar Covalent Bonds:Electronegativity Electrostatic potential maps Show calculated charge distributions Colors indicate electron-rich (red;8-)and electron-poor (blue;δ+)regions (a Methanol,CHaOH,has a polar covalent C-O bond, Oxygen:EN=3.5 Carbon:EN=2.5 H and methyllithium has a Difference=1.0 polar covalent C-Li bond Methanol A crossed arrow+→ is used to indicate direction Carbon:EN 2.5 of bond polarity Lithium:EN 1.0 Electrons are displaced Difference=1.5 in the direction of the arrow MethyllithiumElectrostatic potential maps ▪ Show calculated charge distributions ▪ Colors indicate electron-rich (red; d -) and electron-poor (blue; d +) regions ▪ Methanol, CH3OH, has a polar covalent C-O bond, and methyllithium has a polar covalent C-Li bond ▪ A crossed arrow is used to indicate direction of bond polarity ▪ Electrons are displaced in the direction of the arrow Polar Covalent Bonds: Electronegativity