正在加载图片...
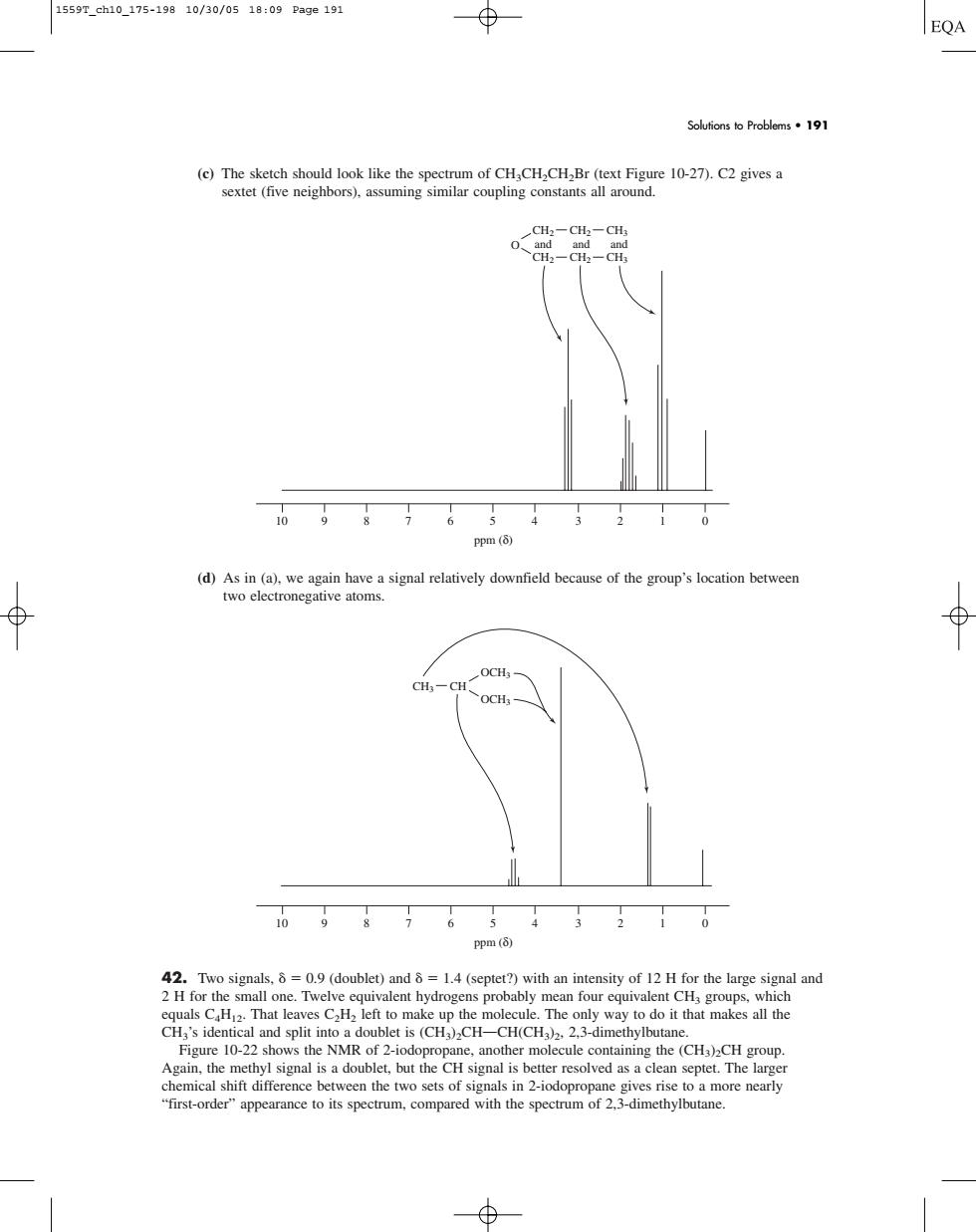
1559reh10175-19g10/30/0518:09Pag0191 Solutions to Probloms191 C:-CH 0,8765432 ppm (5) (d)As in (a). two electronegative atoms. a signal relatively downfield bcause of the group's location between OCH 0987654320 opm (8) ()) equals CThat leaves CH left to make up the molecule.The on y way to do it that makes all the CH,'s identic and split n doublet is (CH)CH- H(CH).2.3-dimet(c) The sketch should look like the spectrum of CH3CH2CH2Br (text Figure 10-27). C2 gives a sextet (five neighbors), assuming similar coupling constants all around. (d) As in (a), we again have a signal relatively downfield because of the group’s location between two electronegative atoms. 42. Two signals, 0.9 (doublet) and 1.4 (septet?) with an intensity of 12 H for the large signal and 2 H for the small one. Twelve equivalent hydrogens probably mean four equivalent CH3 groups, which equals C4H12. That leaves C2H2 left to make up the molecule. The only way to do it that makes all the CH3’s identical and split into a doublet is (CH3)2CHOCH(CH3)2, 2,3-dimethylbutane. Figure 10-22 shows the NMR of 2-iodopropane, another molecule containing the (CH3)2CH group. Again, the methyl signal is a doublet, but the CH signal is better resolved as a clean septet. The larger chemical shift difference between the two sets of signals in 2-iodopropane gives rise to a more nearly “first-order” appearance to its spectrum, compared with the spectrum of 2,3-dimethylbutane. 10 9 8 7 6 5 4 3 2 1 0 ppm (δ) CH3 OCH3 OCH3 CH 10 9 8 7 6 5 4 3 2 1 0 ppm (δ) O CH2 and and and CH2 CH3 CH2 CH2 CH3 Solutions to Problems • 191 1559T_ch10_175-198 10/30/05 18:09 Page 191��