正在加载图片...
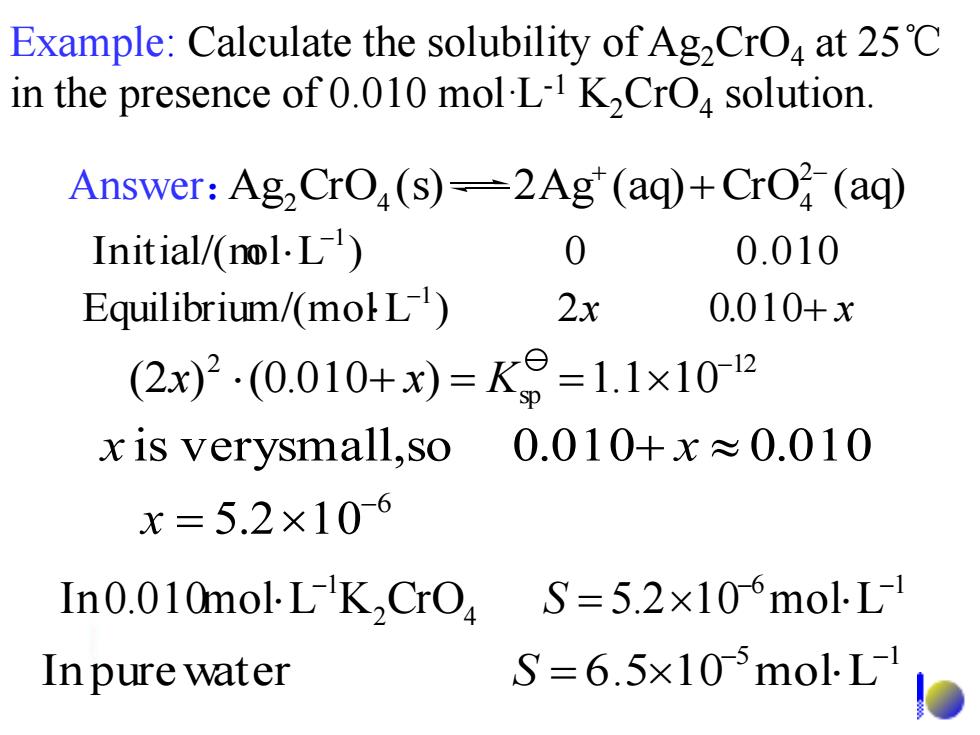
Example:Calculate the solubility of Ag2CrO4 at 25C in the presence of 0.010 mol-L-I K2CrO solution. Answer:Ag2CrO,(s)-2Ag"(aq)+CrO (aq) Initial/(nol.L) 0 0.010 Equilibrium/(moL) 2x 0.010+x (2x)2.(0.010+x)=K9=1.1x102 x is verysmall,so 0.010+x≈0.010 x=5.2×10-6 In0.010mol LK,CrO S=5.2×106molL1 In pure water S=6.5×105molL Example: Calculate the solubility of Ag2CrO4 at 25℃ in the presence of 0.010 mol·L-1 K2CrO4 solution. x . + x - Equilibrium/(mol L ) 2 0 010 1 Initial/(mo l L ) 0 0.010 -1 Answer: Ag CrO (s) 2Ag (aq) CrO (aq) 2 2 4 4 + - + 5 1 In pure water 6.5 1 0 mol L - - S = 6 1 2 4 1 In 0.010mol L K CrO 5.2 1 0 mol L - - - S = 6 5.2 10- x = x is very small,so 0.010+ x 0.010 12 sp 2 (2 ) (0.010 ) 1.1 10- x + x = K =