正在加载图片...
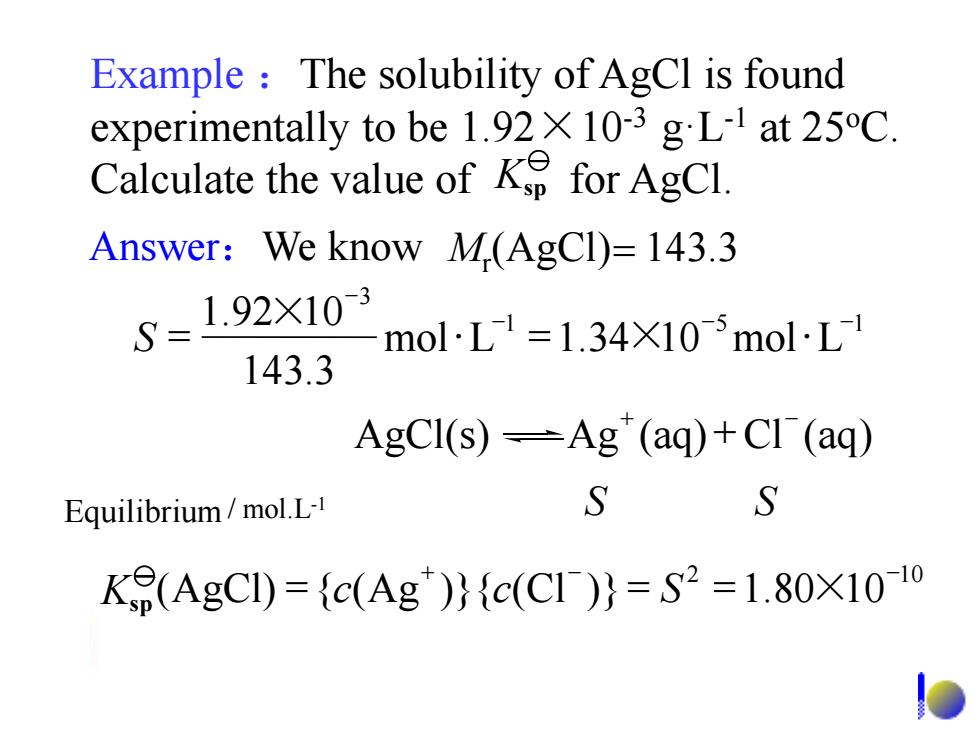
Example The solubility of AgCl is found experimentally to be 1.92X 10-3 gL-1 at 25C. Calculate the value ofK for AgCl. Answer:We know M(AgCl)=143.3 S=1.92X103 molL1=1.34×105molL1 143.3 AgCI(s)=Ag"(aq)+CI (aq) Equilibrium/mol.L- S S K9(AgCI)={c(Ag)}{c(C1)}=S2=1.80X10-1oExample :The solubility of AgCl is found experimentally to be 1.92×10-3 g·L-1 at 25oC. Calculate the value of for AgCl. Equilibrium / mol.L-1 S S Answer:We know Mr (AgCl)= 143.3 1 5 1 3 mol L 1.34 10 mol L 143.3 1.92 10 - - - - S = × = × AgCl(s) Ag (aq) Cl (aq) + - + 2 10 (AgCl) { (Ag )}{ (Cl )} 1.80 10 + - - Ksp = c c = S = × Ksp