正在加载图片...
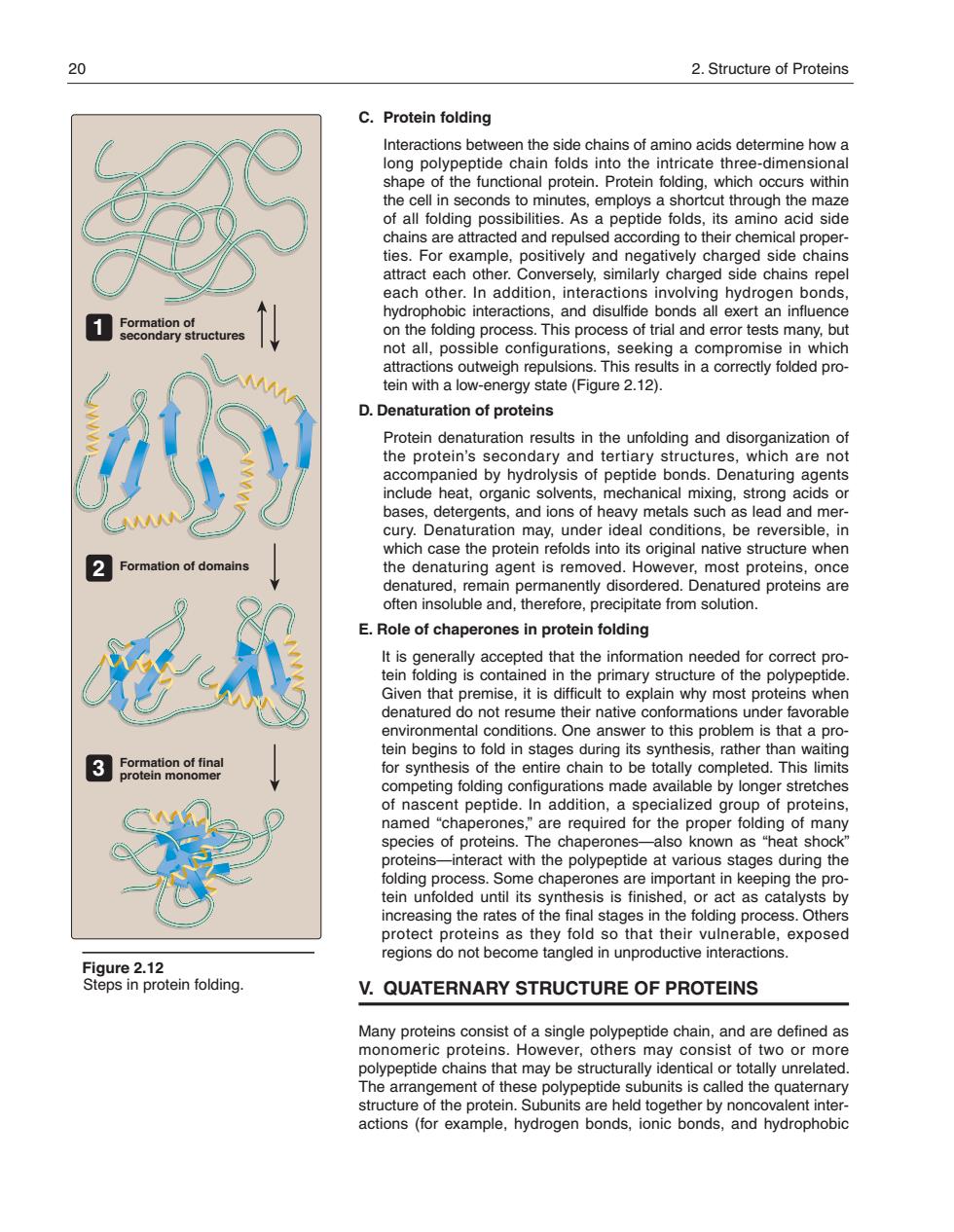
20 2.Structure of Proteins C.Protein folding nteracionsbewentesieocheagesntanheaeicemensoal tein Protein folding which occurs within the cell in seconds to minutes,employs a shortcut through the maze otalodngposbrteAiaPepc9nginiranea id side d side attract each other.Conversely.similarly charged side chains repel on the ol not all.possible configurations.seeking a attractions outweigh repulsions.This results in a correctly folded pro- tein with a low-energy state (Figure 2.12). D.Denaturation of proteins aceompanle8yRadoNysndtpeptd%tocr68natihgar。 nts include heat,organic solvents,mechanical mixing.strong acids or hases,ndetergenisnandonsnatheacetnl such a s lead and mer the denaturing agent is removed.However.most proteins.once E.Role of chaperones in protein folding correct pro denatured do not resume their native conformations under favorable er to this prob is that a pro competing folding configurations made available by longer stretches nascent peptide.. me es of proteins proteins-interact with the polypeptide at various stages during the Ielingnpes.nsmechapeesarasmeariantckeseagesor0y ein un SIS IS I as cat proengohensasoihoytogsoatntherovnerPabie.exposed regions do not become tangled in unproductive interactions. V.QUATERNARY STRUCTURE OF PROTEINS and are define polypeptide chains that may be structurally identical or totally unrelated C. Protein folding Interactions between the side chains of amino acids determine how a long polypeptide chain folds into the intricate three-dimensional shape of the functional protein. Protein folding, which occurs within the cell in seconds to minutes, employs a shortcut through the maze of all folding possibilities. As a peptide folds, its amino acid side chains are attracted and repulsed according to their chemical properties. For example, positively and negatively charged side chains attract each other. Conversely, similarly charged side chains repel each other. In addition, interactions involving hydrogen bonds, hydrophobic interactions, and disulfide bonds all exert an influence on the folding process. This process of trial and error tests many, but not all, possible configurations, seeking a compromise in which attractions outweigh repulsions. This results in a correctly folded protein with a low-energy state (Figure 2.12). D. Denaturation of proteins Protein denaturation results in the unfolding and disorganization of the protein’s secondary and tertiary structures, which are not accompanied by hydrolysis of peptide bonds. Denaturing agents include heat, organic solvents, mechanical mixing, strong acids or bases, detergents, and ions of heavy metals such as lead and mercury. Denaturation may, under ideal conditions, be reversible, in which case the protein refolds into its original native structure when the denaturing agent is removed. However, most proteins, once denatured, remain permanently disordered. Denatured proteins are often insoluble and, therefore, precipitate from solution. E. Role of chaperones in protein folding It is generally accepted that the information needed for correct protein folding is contained in the primary structure of the polypeptide. Given that premise, it is difficult to explain why most proteins when denatured do not resume their native conformations under favorable environmental conditions. One answer to this problem is that a protein begins to fold in stages during its synthesis, rather than waiting for synthesis of the entire chain to be totally completed. This limits competing folding configurations made available by longer stretches of nascent peptide. In addition, a specialized group of proteins, named “chaperones,” are required for the proper folding of many species of proteins. The chaperones—also known as “heat shock” proteins—interact with the polypeptide at various stages during the folding process. Some chaperones are important in keeping the protein unfolded until its synthesis is finished, or act as catalysts by increasing the rates of the final stages in the folding process. Others protect proteins as they fold so that their vulnerable, exposed regions do not become tangled in unproductive interactions. V. QUATERNARY STRUCTURE OF PROTEINS Many proteins consist of a single polypeptide chain, and are defined as monomeric proteins. However, others may consist of two or more polypeptide chains that may be structurally identical or totally unrelated. The arrangement of these polypeptide subunits is called the quaternary structure of the protein. Subunits are held together by noncovalent interactions (for example, hydrogen bonds, ionic bonds, and hydrophobic 20 2. Structure of Proteins Figure 2.12 Formation of secondary structures Formation of domains Formation of final protein monomer Steps in protein folding. 1 2 3 168397_P013-024.qxd7.0:02 Protein structure 5-20-04 2010.4.4 11:31 AM Page 20