正在加载图片...
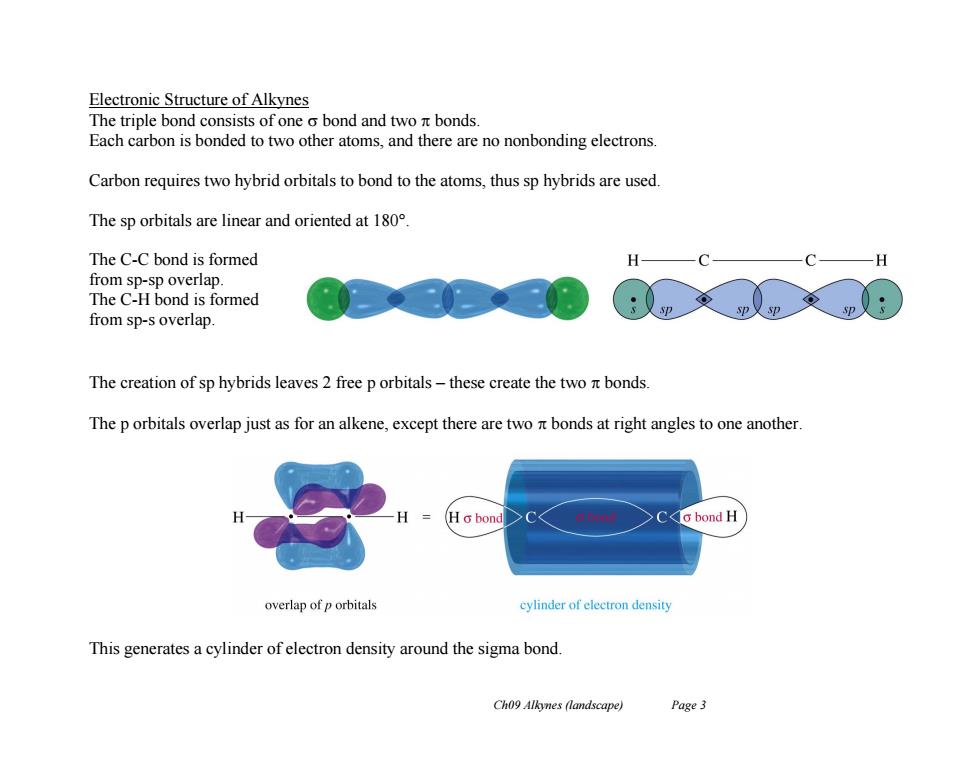
Electronic Structure of Alkynes The triple bond consists of one o bond and two nt bonds. Each carbon is bonded to two other atoms,and there are no nonbonding electrons Carbon requires two hybrid orbitals to bond to the atoms,thus sp hybrids are used. The sp orbitals are linear and oriented at 180 The C-C bond is formed H from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals-these create the two nt bonds The p orbitals overlap just as for an alkene,except there are two nt bonds at right angles to one another. Hc bond o bond H overlap of p orbitals cylinder of eleetron density This generates a cylinder of electron density around the sigma bond. Ch09 Alkynes (landscape) Page 3Ch09 Alkynes (landscape) Page 3 Electronic Structure of Alkynes The triple bond consists of one bond and two bonds. Each carbon is bonded to two other atoms, and there are no nonbonding electrons. Carbon requires two hybrid orbitals to bond to the atoms, thus sp hybrids are used. The sp orbitals are linear and oriented at 180°. The C-C bond is formed from sp-sp overlap. The C-H bond is formed from sp-s overlap. The creation of sp hybrids leaves 2 free p orbitals – these create the two bonds. The p orbitals overlap just as for an alkene, except there are two bonds at right angles to one another. This generates a cylinder of electron density around the sigma bond