正在加载图片...
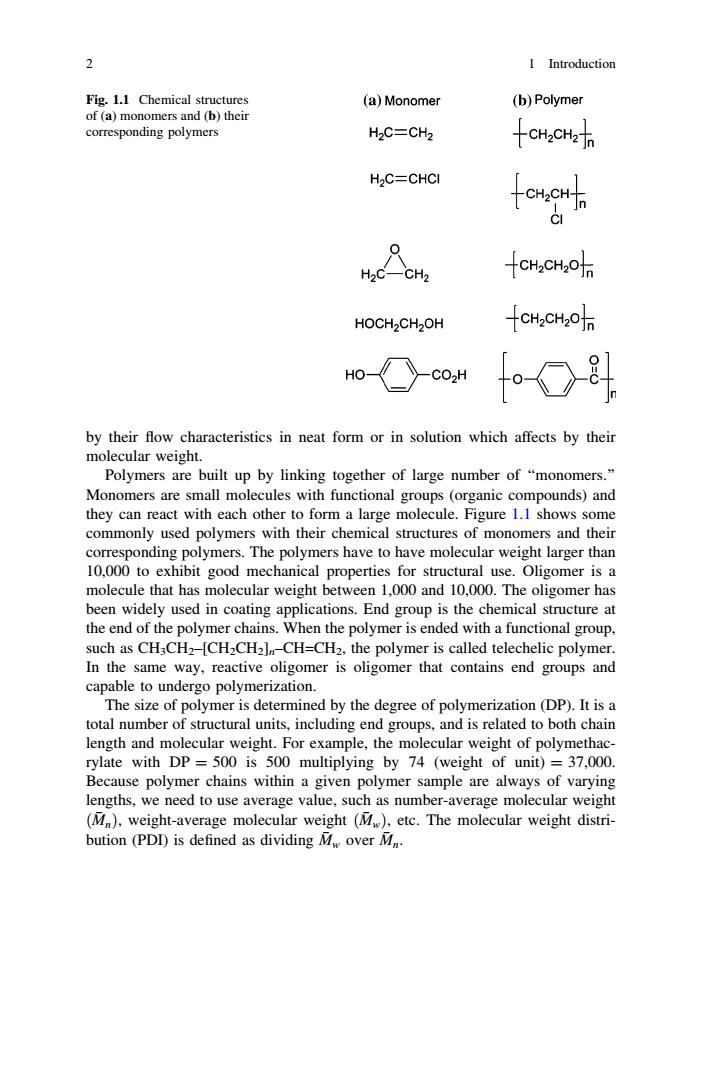
2 I Introduction Fig.1.1 Chemical structures (a)Monomer (b)Polymer of(a)monomers and(b)their corresponding polymers H2C=CH2 十CHaCHz H2C=CHCI H2C—CH2 -CHzCHz0jn HOCH2CH2OH -CH2CH2O f-O斗 by their flow characteristics in neat form or in solution which affects by their molecular weight. Polymers are built up by linking together of large number of "monomers." Monomers are small molecules with functional groups (organic compounds)and they can react with each other to form a large molecule.Figure 1.1 shows some commonly used polymers with their chemical structures of monomers and their corresponding polymers.The polymers have to have molecular weight larger than 10,000 to exhibit good mechanical properties for structural use.Oligomer is a molecule that has molecular weight between 1,000 and 10,000.The oligomer has been widely used in coating applications.End group is the chemical structure at the end of the polymer chains.When the polymer is ended with a functional group, such as CH3CH2-[CH2CH2]-CH=CH2,the polymer is called telechelic polymer. In the same way,reactive oligomer is oligomer that contains end groups and capable to undergo polymerization. The size of polymer is determined by the degree of polymerization(DP).It is a total number of structural units,including end groups,and is related to both chain length and molecular weight.For example,the molecular weight of polymethac- rylate with DP =500 is 500 multiplying by 74 (weight of unit)=37,000. Because polymer chains within a given polymer sample are always of varying lengths,we need to use average value,such as number-average molecular weight (M),weight-average molecular weight(Mw),etc.The molecular weight distri- bution (PDD)is defined as dividing Mw over Mn.by their flow characteristics in neat form or in solution which affects by their molecular weight. Polymers are built up by linking together of large number of ‘‘monomers.’’ Monomers are small molecules with functional groups (organic compounds) and they can react with each other to form a large molecule. Figure 1.1 shows some commonly used polymers with their chemical structures of monomers and their corresponding polymers. The polymers have to have molecular weight larger than 10,000 to exhibit good mechanical properties for structural use. Oligomer is a molecule that has molecular weight between 1,000 and 10,000. The oligomer has been widely used in coating applications. End group is the chemical structure at the end of the polymer chains. When the polymer is ended with a functional group, such as CH3CH2–[CH2CH2]n–CH=CH2, the polymer is called telechelic polymer. In the same way, reactive oligomer is oligomer that contains end groups and capable to undergo polymerization. The size of polymer is determined by the degree of polymerization (DP). It is a total number of structural units, including end groups, and is related to both chain length and molecular weight. For example, the molecular weight of polymethacrylate with DP = 500 is 500 multiplying by 74 (weight of unit) = 37,000. Because polymer chains within a given polymer sample are always of varying lengths, we need to use average value, such as number-average molecular weight Mð Þn , weight-average molecular weight Mð Þ w , etc. The molecular weight distribution (PDI) is defined as dividing M w over M n. Fig. 1.1 Chemical structures of (a) monomers and (b) their corresponding polymers 2 1 Introduction����