正在加载图片...
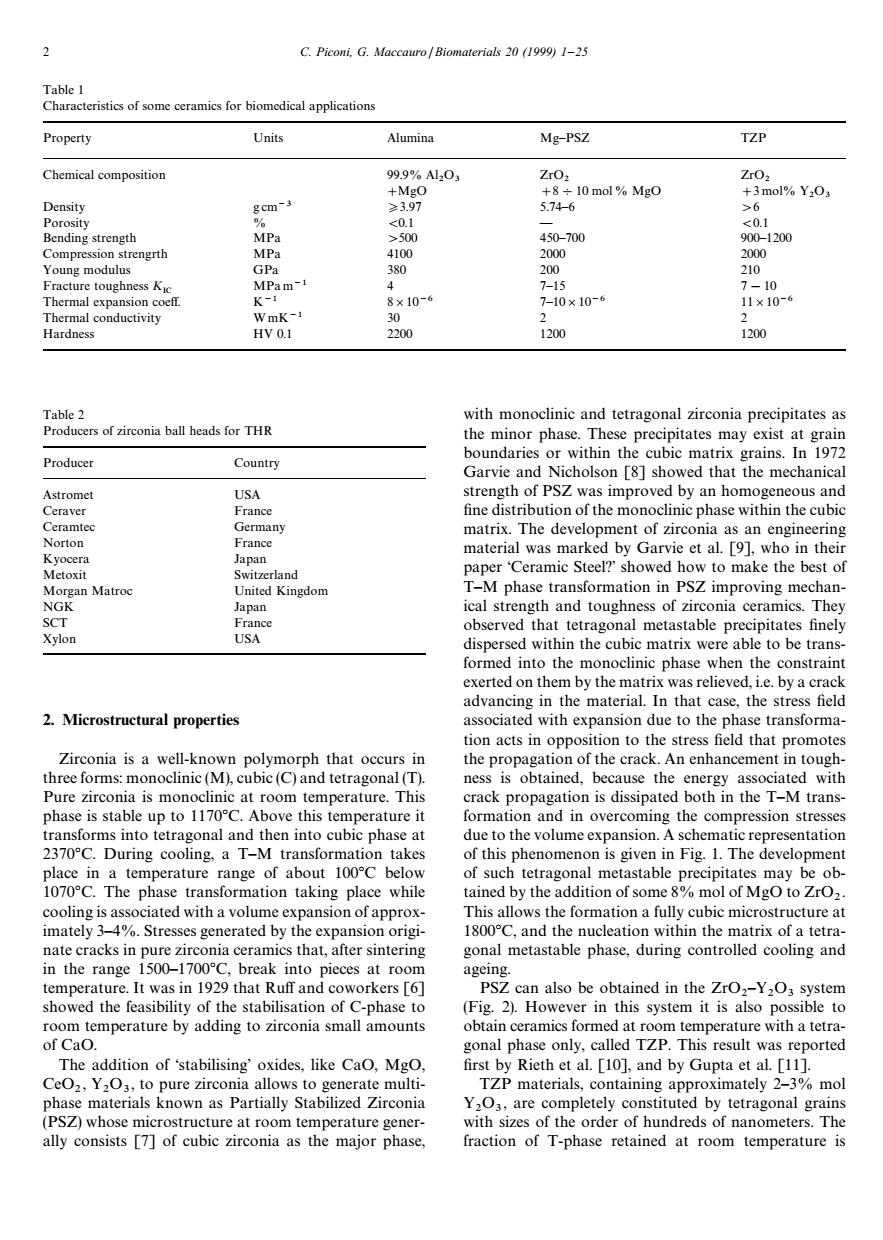
2 C.Piconi.G.Maccauro/Biomaterials 20(1999)1-25 Property Units Alumina Mg-PSZ TZP Chemical 9%A0, 450-700 1200 engrth 2000 re toughn css K 7-10 1x10- ardness 1200 1200 with monoclinic and tetragonal zirconia precipitates as Producers of zirconia ball heads for THR the minor phase.These precipitates may exist at grain Producer Country boundaries or within the cubic matrix grains.In 1972 Garvie anc holson [8]sh wed that the mechanica Astrome USA of the Ceramte Germany matrix.The development of zirconia as an engineering material was marked by Garvie et al.[9],who in their paper owed how to make the best of tion in PS Z improving mecn observed that tetrago nal metastable p dispersed within the cubic matrix were able to be trans forme into the monoclinic phase v e m e by a cra 2.Microstructural properties associated with ex nsion due to the tion acts in opposition to the stress field that promotes Zirconia is a well-known polymorph that occurs in hree forms:monoclinic(M),cubic(C)and tetragonal(T) es Because the propa ransforms into tetragonal and then into cubic phase at due to the volume nsion.A schematic representation 2370C.During cooling.a T-M transformation takes of this phenomenon is given in Fig.1.The development ol suc tetrago I metastable may ob ing plac on ol s of Mgo to ZrO imat matrix of a tetra nate cracks in pure zirconia ceramics that,after sintering gonal metastable phase.during controlled cooling and in the range 1500-1700C,break into pieces at roo ageing. s1n92 that n and coworkers [6 also be ob ined in the ZrC aoe时adin trm ed a ure with a tetra of Cao onal phase only.called TZP.This result was reported The addition of'stabilising'oxides,like CaO.Mgo. first by Rieth et al[]and by Gupta et al.[11] TZP materials,containing approximately 2-3 %m0 (PSZt d by tetragonal ra fraction of T-phase retained at room temperature is Table 1 Characteristics of some ceramics for biomedical applications Property Units Alumina Mg—PSZ TZP Chemical composition 99.9% Al2 O3 ZrO2 ZrO2 #MgO #8%10 mol% MgO #3 mol% Y2 O3 Density g cm~3 *3.97 5.74—6 '6 Porosity % (0.1 — (0.1 Bending strength MPa '500 450—700 900—1200 Compression strengrth MPa 4100 2000 2000 Young modulus GPa 380 200 210 Fracture toughness KIC MPa m~1 4 7—15 7!10 Thermal expansion coeff. K~1 8]10~6 7—10]10~6 11]10~6 Thermal conductivity W mK~1 30 2 2 Hardness HV 0.1 2200 1200 1200 Table 2 Producers of zirconia ball heads for THR Producer Country Astromet USA Ceraver France Ceramtec Germany Norton France Kyocera Japan Metoxit Switzerland Morgan Matroc United Kingdom NGK Japan SCT France Xylon USA 2. Microstructural properties Zirconia is a well-known polymorph that occurs in three forms: monoclinic (M), cubic (C) and tetragonal (T). Pure zirconia is monoclinic at room temperature. This phase is stable up to 1170°C. Above this temperature it transforms into tetragonal and then into cubic phase at 2370°C. During cooling, a T—M transformation takes place in a temperature range of about 100°C below 1070°C. The phase transformation taking place while cooling is associated with a volume expansion of approximately 3—4%. Stresses generated by the expansion originate cracks in pure zirconia ceramics that, after sintering in the range 1500—1700°C, break into pieces at room temperature. It was in 1929 that Ruff and coworkers [6] showed the feasibility of the stabilisation of C-phase to room temperature by adding to zirconia small amounts of CaO. The addition of ‘stabilising’ oxides, like CaO, MgO, CeO2 , Y2 O3 , to pure zirconia allows to generate multiphase materials known as Partially Stabilized Zirconia (PSZ) whose microstructure at room temperature generally consists [7] of cubic zirconia as the major phase, with monoclinic and tetragonal zirconia precipitates as the minor phase. These precipitates may exist at grain boundaries or within the cubic matrix grains. In 1972 Garvie and Nicholson [8] showed that the mechanical strength of PSZ was improved by an homogeneous and fine distribution of the monoclinic phase within the cubic matrix. The development of zirconia as an engineering material was marked by Garvie et al. [9], who in their paper ‘Ceramic Steel?’ showed how to make the best of T—M phase transformation in PSZ improving mechanical strength and toughness of zirconia ceramics. They observed that tetragonal metastable precipitates finely dispersed within the cubic matrix were able to be transformed into the monoclinic phase when the constraint exerted on them by the matrix was relieved, i.e. by a crack advancing in the material. In that case, the stress field associated with expansion due to the phase transformation acts in opposition to the stress field that promotes the propagation of the crack. An enhancement in toughness is obtained, because the energy associated with crack propagation is dissipated both in the T—M transformation and in overcoming the compression stresses due to the volume expansion. A schematic representation of this phenomenon is given in Fig. 1. The development of such tetragonal metastable precipitates may be obtained by the addition of some 8% mol of MgO to ZrO2 . This allows the formation a fully cubic microstructure at 1800°C, and the nucleation within the matrix of a tetragonal metastable phase, during controlled cooling and ageing. PSZ can also be obtained in the ZrO2 —Y2 O3 system (Fig. 2). However in this system it is also possible to obtain ceramics formed at room temperature with a tetragonal phase only, called TZP. This result was reported first by Rieth et al. [10], and by Gupta et al. [11]. TZP materials, containing approximately 2—3% mol Y2 O3 , are completely constituted by tetragonal grains with sizes of the order of hundreds of nanometers. The fraction of T-phase retained at room temperature is 2 C. Piconi, G. Maccauro / Biomaterials 20 (1999) 1—25