正在加载图片...
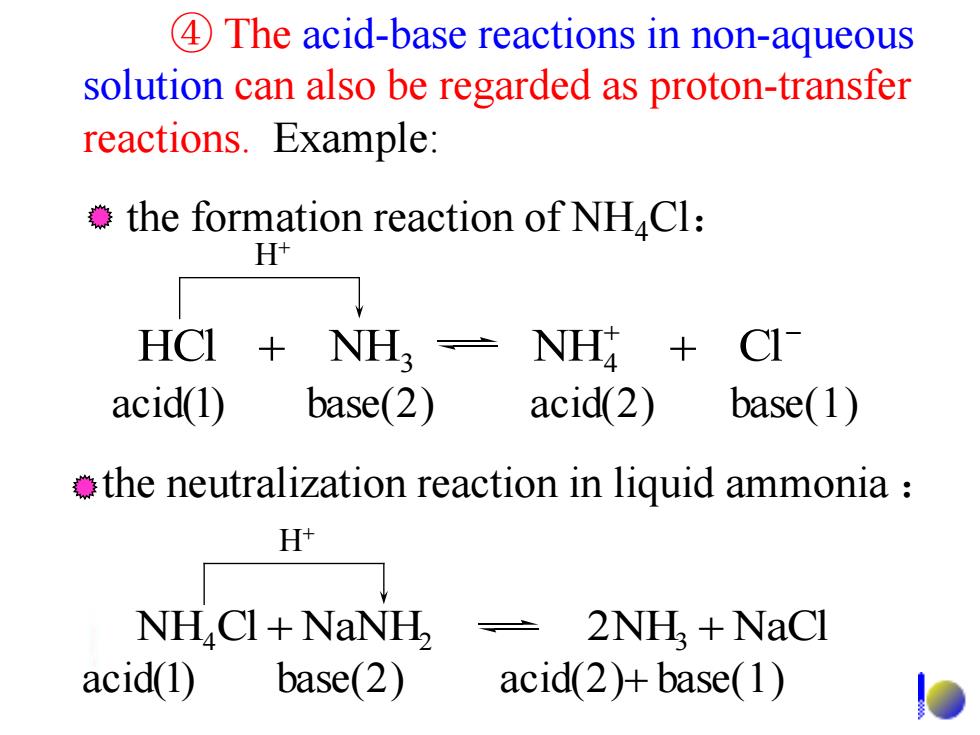
4 The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions.Example: the formation reaction of NHCl: H+ HCI NH- NH acid(1) base(2) acid(2) base(1) the neutralization reaction in liquid ammonia H NH CI+NaNH 2NH+NaCl acid(1) base(2) acid(2)+base(1) ④ The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions. Example: the formation reaction of NH4Cl: H+ the neutralization reaction in liquid ammonia : H+ + - HCl + NH NH + Cl 3 4 NH Cl NaNH 2NH NaCl 4 + 2 3 + acid(1) base(2) acid(2) base(1) acid(1) base(2) acid(2)+ base(1)