正在加载图片...
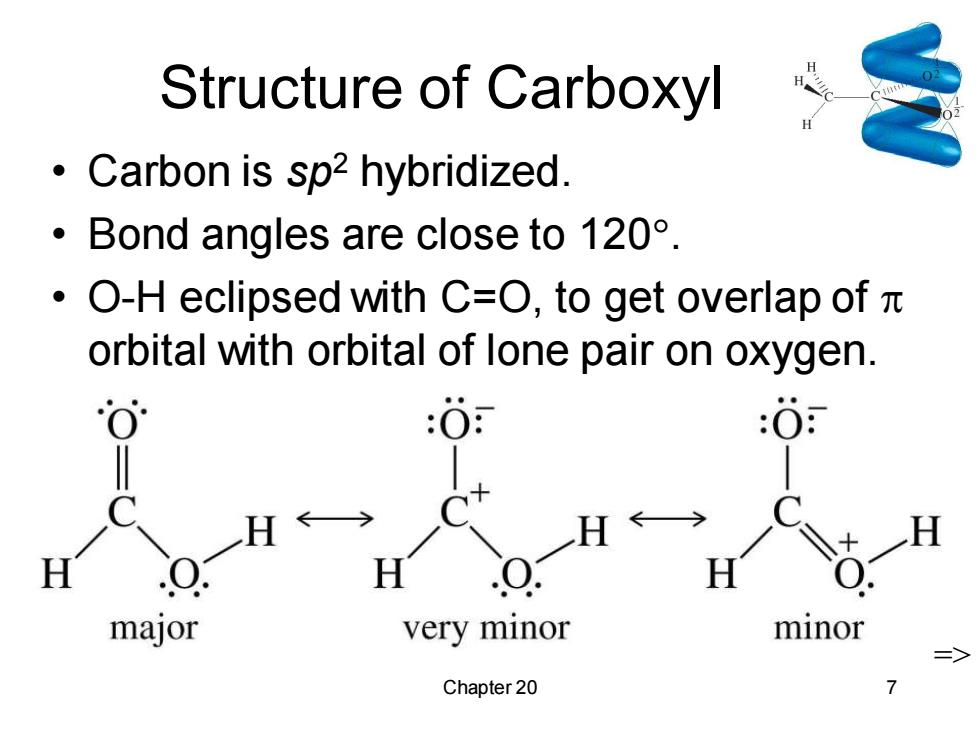
Structure of Carboxyl Carbon is sp2 hybridized. ·Bond angles are close to120°. ·O-H eclipsed with C=O,to get overlap ofπ orbital with orbital of lone pair on oxygen. :O major very minor minor Chapter 20Chapter 20 7 Structure of Carboxyl • Carbon is sp2 hybridized. • Bond angles are close to 120. • O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. =>