正在加载图片...
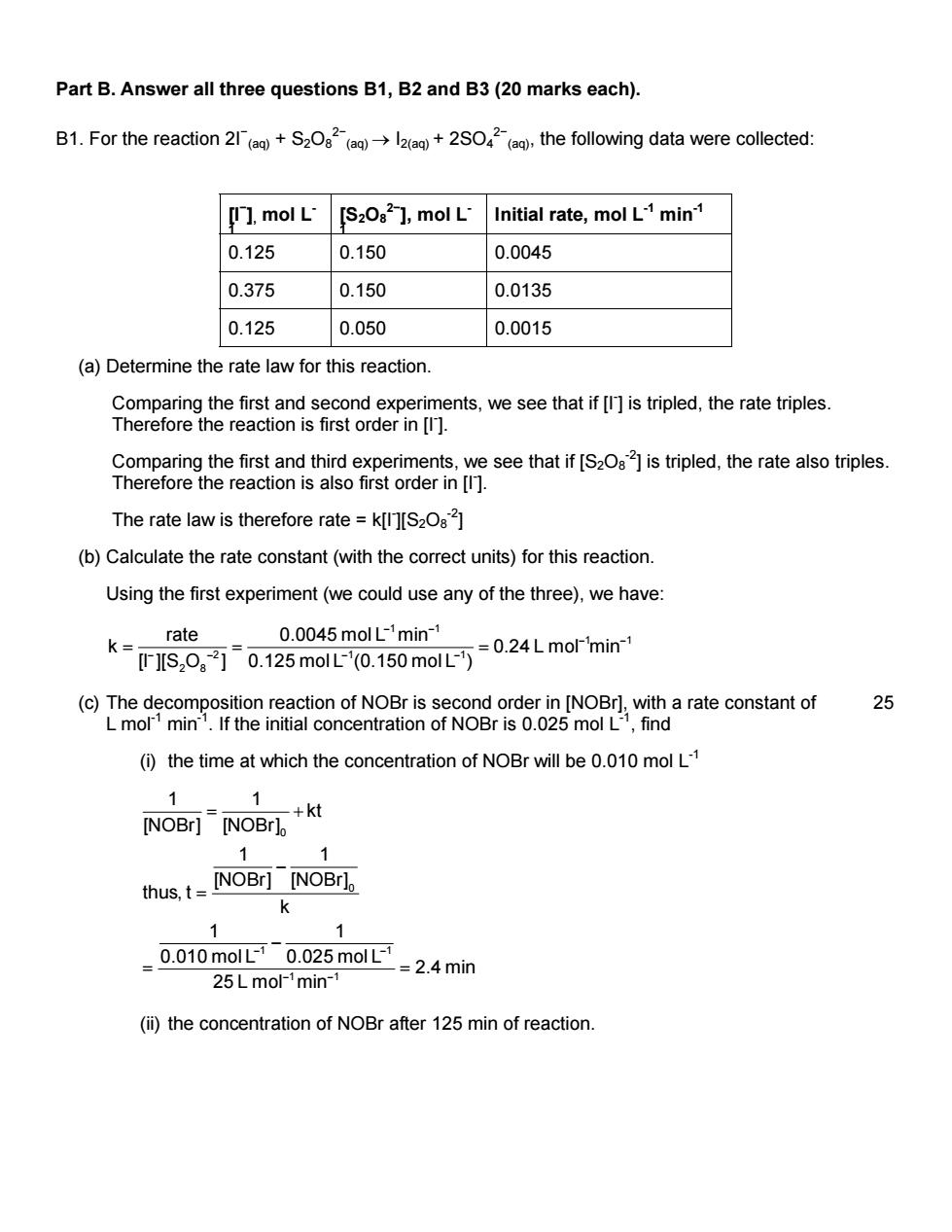
Part B.Answer all three questions B1,B2 and B3(20 marks each). B1.For the reaction 2+S2O+2SO()the following data were collected: [I ]mol L'[S2O82 ]mol L'Initial rate,mol L min 0.125 0.150 0.0045 0.375 0.150 0.0135 0.125 0.050 0.0015 (a)Determine the rate law for this reaction. eoeaeC8ms8scoe6mens,uesaehatf们stpled,heaepes The Comparing the first and third experiments,we see that if [S2Oa]is tripled,the rate also triples. Therefore the reaction is also first order in [ The rate law is therefore rate =k[][S2O8] (b)Calculate the rate constant(with the correct units)for this reaction. Using the first experiment(we could use any of the three),we have: rate 0.0045 mol L-'min- .0.125m.150mLmor'min (c)The decomposition reaction of NOBr is second order in [NOBr].with a rate constant of 25 L mol1 min1.If the initial concentration of NOBr is 0.025 mol L-1,find (i)the time at which the concentration of NOBr will be 0.010 mol L 1 NOBr]"NOBri,+ thus,t=[NOBr][NOBr), k 1 1 0.010 mol L-1 0.025 mol L-1 =2.4min 25 L mol-min (ii)the concentration of NOBr after 125 min of reaction. Part B. Answer all three questions B1, B2 and B3 (20 marks each). B1. For the reaction 2I− (aq) + S2O8 2− (aq) → I2(aq) + 2SO4 2− (aq), the following data were collected: [I− ], mol L- 1 [S2O8 2− ], mol L- 1 Initial rate, mol L-1 min-1 0.125 0.150 0.0045 0.375 0.150 0.0135 0.125 0.050 0.0015 (a) Determine the rate law for this reaction. Comparing the first and second experiments, we see that if [I- ] is tripled, the rate triples. Therefore the reaction is first order in [I- ]. Comparing the first and third experiments, we see that if [S2O8 -2] is tripled, the rate also triples. Therefore the reaction is also first order in [I- ]. The rate law is therefore rate = k[I- ][S2O8 -2] (b) Calculate the rate constant (with the correct units) for this reaction. Using the first experiment (we could use any of the three), we have: 1 1 1 1 2 11 2 8 rate 0.0045 mol L min k 0 [I ][S O ] 0.125 mol L (0.150 mol L ) − − .24 L mol min − − − − − − = = = (c) The decomposition reaction of NOBr is second order in [NOBr], with a rate constant of 25 L mol-1 min-1. If the initial concentration of NOBr is 0.025 mol L-1, find (i) the time at which the concentration of NOBr will be 0.010 mol L-1 0 0 1 1 1 1 1 1 kt [NOBr] [NOBr] 1 1 [NOBr] [NOBr] thus, t k 1 1 0.010 mol L 0.025 mol L 2.4 min 25 L mol min − − − − = + − = − = = (ii) the concentration of NOBr after 125 min of reaction