正在加载图片...
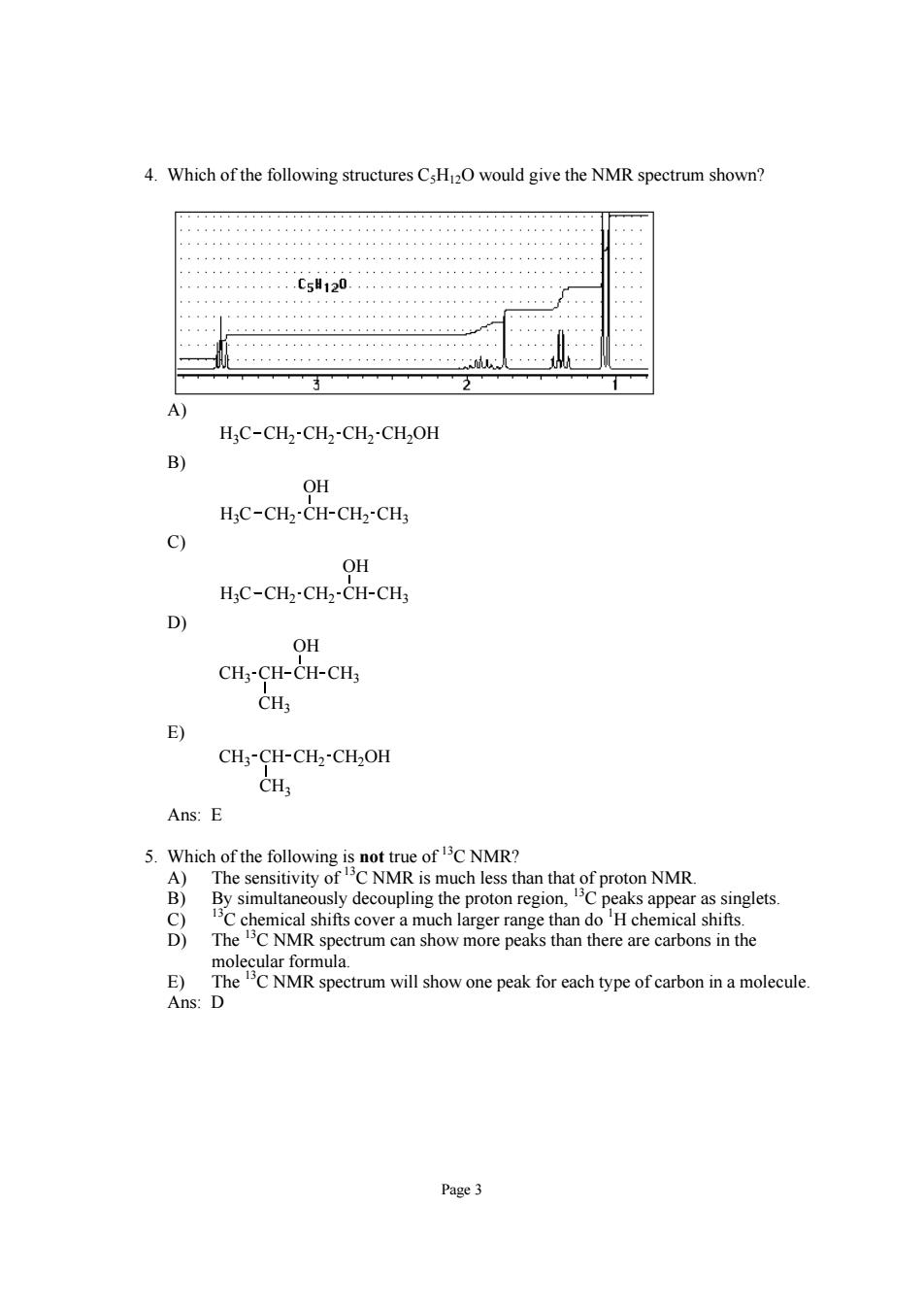
4.Which of the following structures would give the NMR spectrum shown? c5120 H:C-CH2-CH2-CH2-CH2OH B OH H;C-CH2-CH-CH2-CH 9 OH HC-CH2-CH2-CH-CH 9 OH CH3-CH-CH-CH3 CH E) CH;-CH-CH2-CH2OH Ans:E 5.Which of the following is not true of C NMR? A)The sensitivity ofC NMR is much less than that of proton NMR. B)By simultaneously decoupling the proton region,Cpeaks appear as singlets. The chemical shifts cover a much larger range than doH chemical shifts. wmore peaks hanrerc E Ans:D spectrum will show one peak for each type of carbon in a molecule Page3 Page 3 4. Which of the following structures C5H12O would give the NMR spectrum shown? A) H3C CH2 CH2 CH2 CH2OH B) H3C CH2 CH CH2 CH3 OH C) H3C CH2 CH2 CH CH3 OH D) CH3 CH3 CH CH CH3 OH E) CH3 CH3 CH CH2 CH2OH Ans: E 5. Which of the following is not true of 13C NMR? A) The sensitivity of 13C NMR is much less than that of proton NMR. B) By simultaneously decoupling the proton region, 13C peaks appear as singlets. C) 13C chemical shifts cover a much larger range than do 1 H chemical shifts. D) The 13C NMR spectrum can show more peaks than there are carbons in the molecular formula. E) The 13C NMR spectrum will show one peak for each type of carbon in a molecule. Ans: D