正在加载图片...
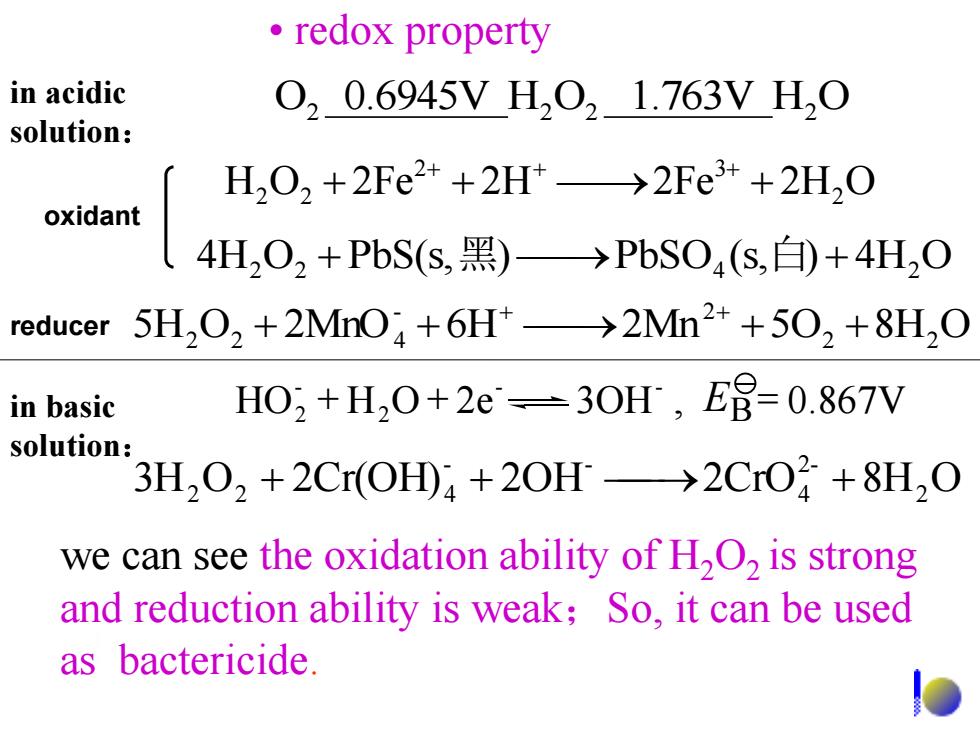
·redox property in acidic 020.6945VH021.763VH0 solution: H202+2Fe2++2Ht→2Fe3++2H,0 oxidant 4H202+PbS(s,黑)→PbS04(s,白+4H2O reducer 5H,02+2MnO4+6H→2Mn2++502+8H,0 in basic H02+H,0+2e=3OH,E8=0.867V solution 3H,O,+2Cr(OH)a +20H>2CrO+8H,O we can see the oxidation ability of H2O2 is strong and reduction ability is weak;So,it can be used as bactericide. we can see the oxidation ability of H2O2 is strong and reduction ability is weak;So, it can be used as bactericide. 3H O 2Cr(OH) 2OH 2CrO 8H2 O 2- 4 - - 2 2 + 4 + + H O 2Fe 2H 2Fe 2H2 O 2 3 2 2 + + + + + + in acidic solution: O2 0.6945V H2 O2 1.763V H2 O • redox property HO H O 2e 3OH , 0.867V - - 2 - 2 + + E = B 5H O 2MnO 6H 2Mn 5O2 8H2 O - 2 2 2 + 4 + + + + + 4H2 O2 + PbS(s,黑) PbSO4 (s,白) + 4H2 O in basic solution: oxidant reducer