正在加载图片...
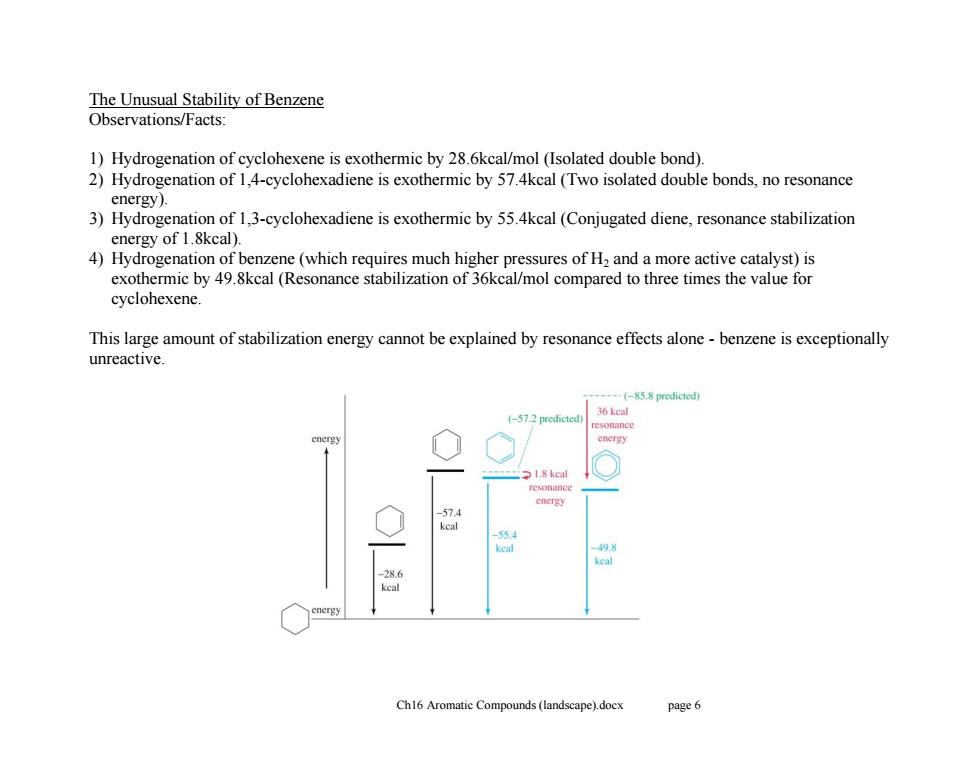
The Unusual Stability of Benzene Observations/Facts: 1)Hydrogenation of cyclohexene is exothermic by 28.6kcal/mol (Isolated double bond). 2)Hydrogenation of 1,4-cyclohexadiene is exothermic by 57.4kcal(Two isolated double bonds,no resonance energy). 3)Hydrogenation of 1,3-cyclohexadiene is exothermic by 55.4kcal(Conjugated diene,resonance stabilization energy of 1.8kcal). 4)Hydrogenation of benzene(which requires much higher pressures of H2 and a more active catalyst)is exothermic by 49.8kcal (Resonance stabilization of 36kcal/mol compared to three times the value for cyclohexene This large amount of stabilization energy cannot be explained by resonance effects alone-benzene is exceptionally unreactive. (-85.8 predictedy 36 kcal (-57.2 predicted) 1.8 kcal 55 keal 28.6 kcal Ch16 Aromatic Compounds (landscape).docx page 6Ch16 Aromatic Compounds (landscape).docx page 6 The Unusual Stability of Benzene Observations/Facts: 1) Hydrogenation of cyclohexene is exothermic by 28.6kcal/mol (Isolated double bond). 2) Hydrogenation of 1,4-cyclohexadiene is exothermic by 57.4kcal (Two isolated double bonds, no resonance energy). 3) Hydrogenation of 1,3-cyclohexadiene is exothermic by 55.4kcal (Conjugated diene, resonance stabilization energy of 1.8kcal). 4) Hydrogenation of benzene (which requires much higher pressures of H2 and a more active catalyst) is exothermic by 49.8kcal (Resonance stabilization of 36kcal/mol compared to three times the value for cyclohexene. This large amount of stabilization energy cannot be explained by resonance effects alone - benzene is exceptionally unreactive