正在加载图片...
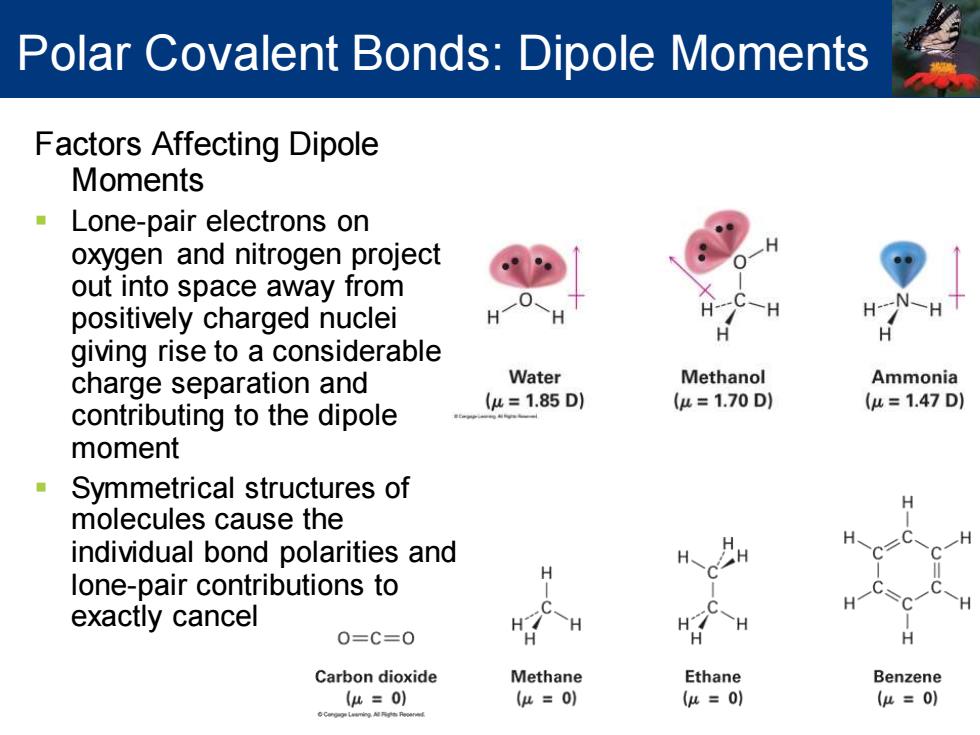
Polar Covalent Bonds:Dipole Moments Factors Affecting Dipole Moments Lone-pair electrons on oxygen and nitrogen project out into space away from positively charged nuclei giving rise to a considerable charge separation and Water Methanol Ammonia contributing to the dipole (u=1.85D) (u=1.70D1 (u=1.47D) moment Symmetrical structures of molecules cause the individual bond polarities and lone-pair contributions to H exactly cancel H H 0=C=0 H Carbon dioxide Methane Ethane Benzene u=0) (μ=0) (u=0) (μ=0) Polar Covalent Bonds: Dipole Moments Factors Affecting Dipole Moments ▪ Lone-pair electrons on oxygen and nitrogen project out into space away from positively charged nuclei giving rise to a considerable charge separation and contributing to the dipole moment ▪ Symmetrical structures of molecules cause the individual bond polarities and lone-pair contributions to exactly cancel