正在加载图片...
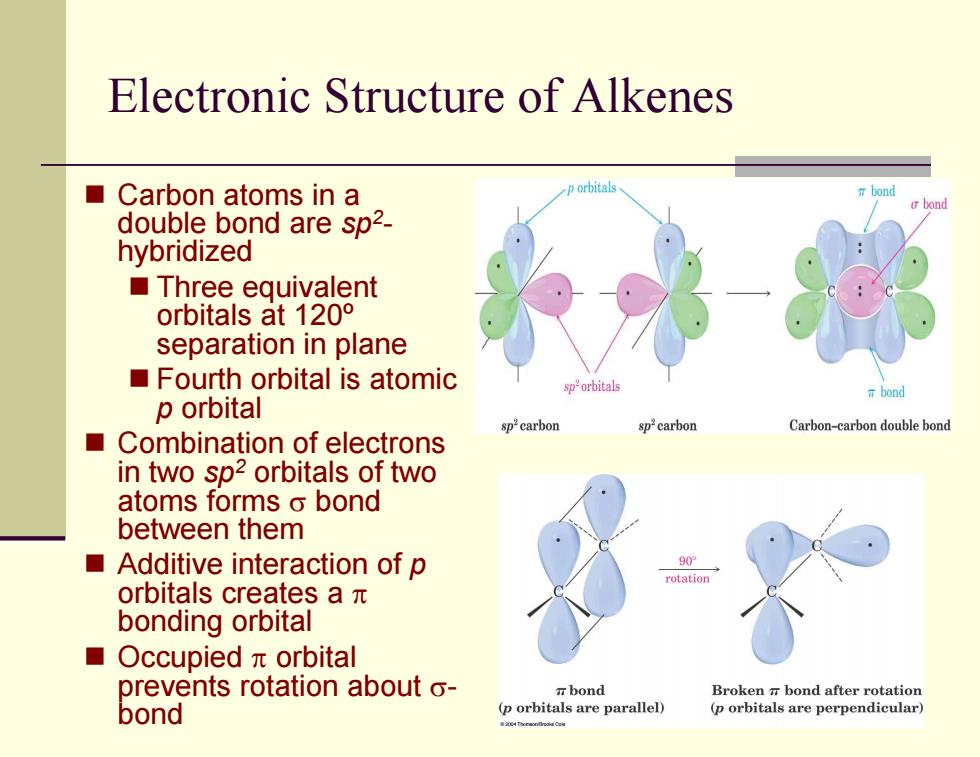
Electronic Structure of Alkenes ■Carbon atoms in a p orbitals bond double bond are sp2- hybridized ■Three equivalent orbitals at120° separation in plane Fourth orbital is atomic orbitals T bond p orbital sp2carbon Carbon-carbon double bond Combination of electrons in two sp2 orbitals of two atoms forms o bond between them Additive interaction of p 90 rotatio orbitals creates aπ bonding orbital ■ Occupiedπorbital prevents rotation about o- bond Broken bond after rotation bond (p orbitals are parallel) (p orbitals are perpendicular) Electronic Structure of Alkenes Carbon atoms in a double bond are sp 2 - hybridized Three equivalent orbitals at 120º separation in plane Fourth orbital is atomic p orbital Combination of electrons in two sp 2 orbitals of two atoms forms σ bond between them Additive interaction of p orbitals creates a π bonding orbital Occupied π orbital prevents rotation about σ - bond