正在加载图片...
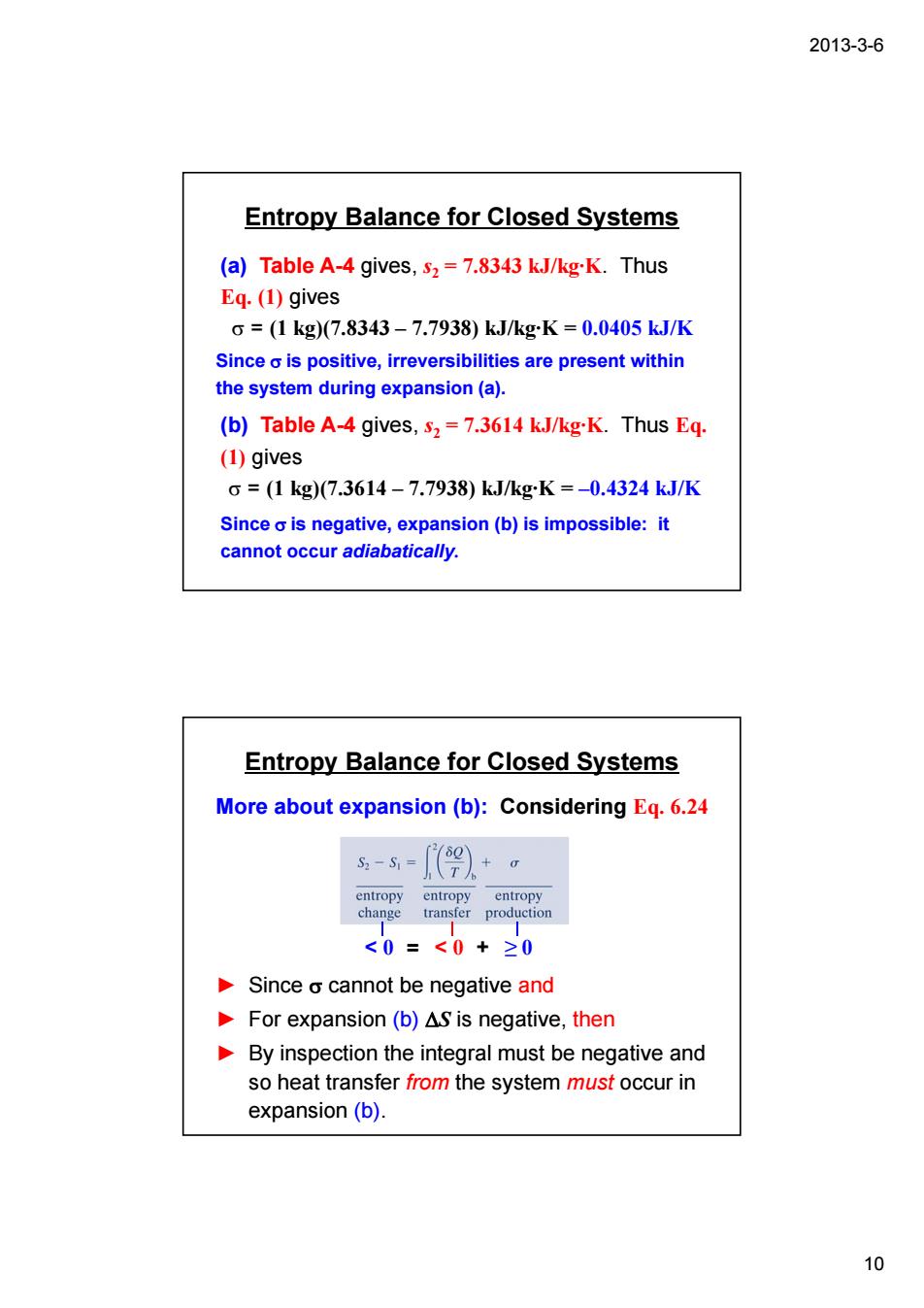
2013-3-6 Entropy Balance for Closed Systems (a)Table A-4 gives,s2=7.8343 kJ/kg-K.Thus Eq.(1)gives o=(1kg)7.8343-7.7938)kJ/kgK=0.0405kJ/K Sinceo is positive,irreversibilities are present within the system during expansion(a). (b)Table A-4 gives,s2=7.3614 k.J/kg-K.Thus Eq (1)gives c=(1kg)(7.3614-7.7938)kJ/kgK=-0.4324kJ/K Since o is negative,expansion(b)is impossible:it cannot occur adiabatically. Entropy Balance for Closed Systems More about expansion(b):Considering Eq.6.24 s-5=9)+。 <0=<0+20 Since o cannot be negative and For expansion(b)AS is negative,then By inspection the integral must be negative and so heat transfer from the system must occur in expansion(b). 10 2013-3-6 10 Entropy Balance for Closed Systems (a) Table A-4 gives, s2 = 7.8343 kJ/kg·K. Thus Eq. (1) gives σ = (1 kg)(7.8343 – 7.7938) kJ/kg·K = 0.0405 kJ/K Since σ is positive, irreversibilities are present within the system during expansion (a). (b) Table A-4 gives, s2 = 7.3614 kJ/kg·K. Thus Eq. (1) gives σ = (1 kg)(7.3614 – 7.7938) kJ/kg·K = –0.4324 kJ/K Since σ is negative, expansion (b) is impossible: it cannot occur adiabatically. Entropy Balance for Closed Systems ► Since σ cannot be negative and ► For expansion (b) ΔS is negative, then ► By inspection the integral must be negative and so heat transfer from the system must occur in expansion (b). More about expansion (b): Considering Eq. 6.24 < 0 = < 0 + ≥ 0