正在加载图片...
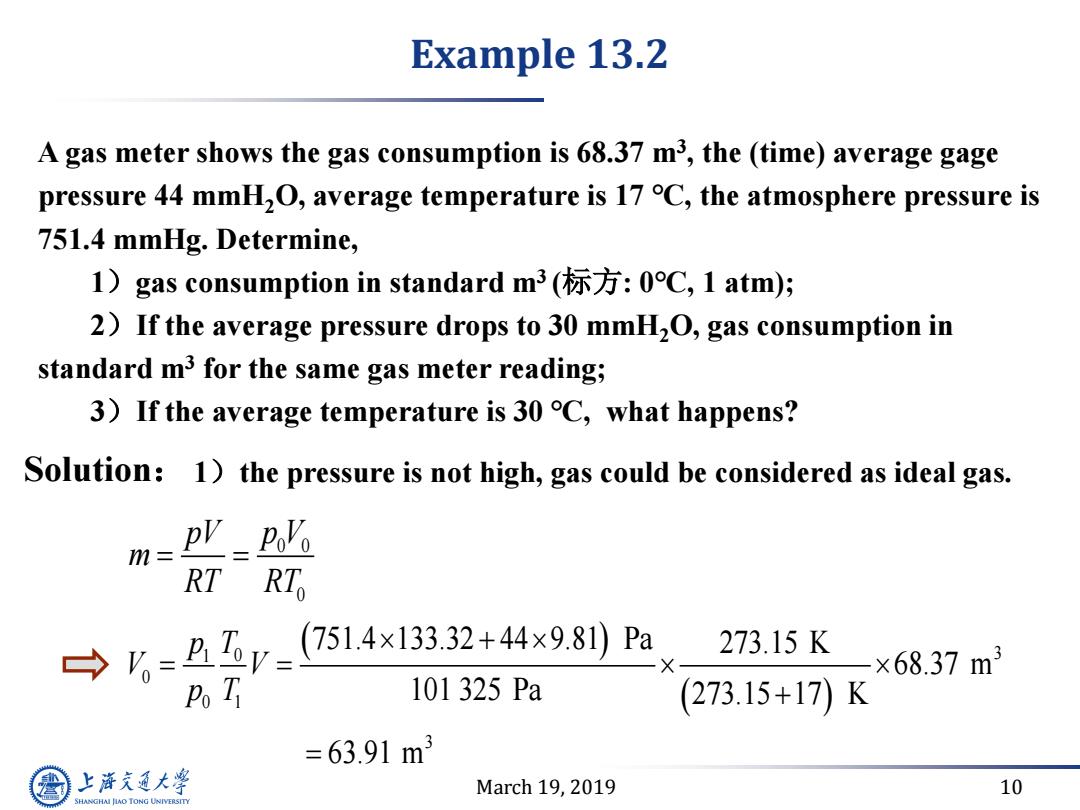
Example 13.2 A gas meter shows the gas consumption is 68.37 m3,the (time)average gage pressure 44 mmH2O,average temperature is 17 C,the atmosphere pressure is 751.4 mmHg.Determine, 1)gas consumption in standard m3(标方:0C,1atm); 2)If the average pressure drops to 30 mmH2O,gas consumption in standard m3 for the same gas meter reading; 3)If the average temperature is 30 C,what happens? Solution:1)the pressure is not high,gas could be considered as ideal gas. m=p'-=2% RT RTo →=Br=(7514x133.32+44×981Pa 273.15K ×68.37m3 Po Ti 101325Pa (273.15+17)K =63.91m3 上游充通大 March 19,2019 10 SHANGHAI JIAO TONG UNIVERSITYMarch 19, 2019 10 A gas meter shows the gas consumption is 68.37 m3 , the (time) average gage pressure 44 mmH2O, average temperature is 17 ℃, the atmosphere pressure is 751.4 mmHg. Determine, 1)gas consumption in standard m3 (标方: 0℃, 1 atm); 2)If the average pressure drops to 30 mmH2O, gas consumption in standard m3 for the same gas meter reading; 3)If the average temperature is 30 ℃, what happens? 1)the pressure is not high, gas could be considered as ideal gas. 0 0 0 pV p V m RT RT Solution: 1 0 3 0 0 1 3 751.4 133.32 44 9.81 Pa 273.15 K 68.37 m 101 325 Pa 273.15 17 K 63.91 m p T V V p T Example 13.2