正在加载图片...
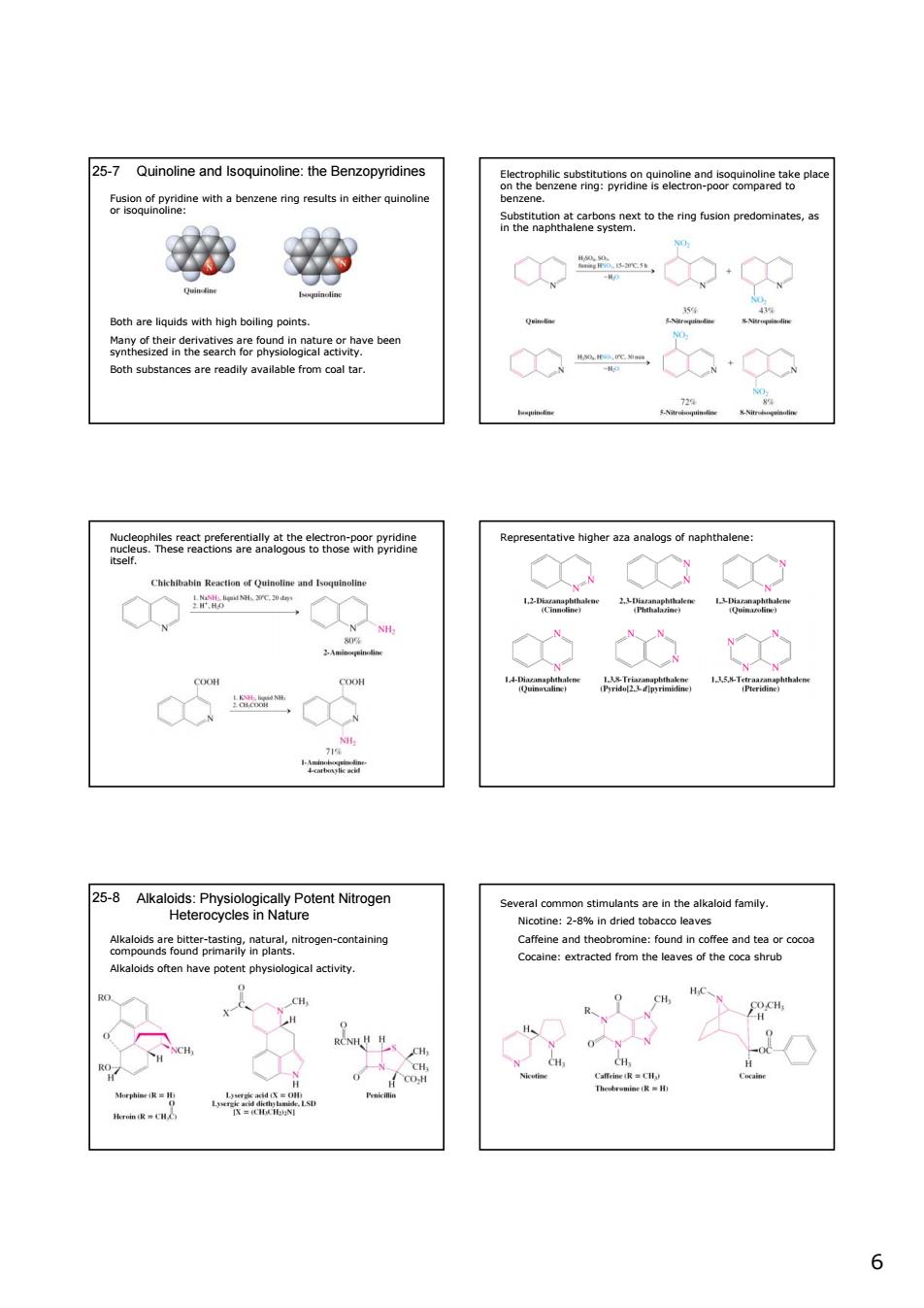
25-7 Quinoline and soquinoine:the Benzopyridines ao6seaa6a2heepa withbnginth Botharequsthigh boing points. 他物 2-Akaod enirogen apetarorps3,mttogencomtainy tent ab siological activity 人又g 66 25-7 Quinoline and Isoquinoline: the Benzopyridines Fusion of pyridine with a benzene ring results in either quinoline or isoquinoline: Both are liquids with high boiling points. Many of their derivatives are found in nature or have been synthesized in the search for physiological activity. Both substances are readily available from coal tar. Electrophilic substitutions on quinoline and isoquinoline take place on the benzene ring: pyridine is electron-poor compared to benzene. Substitution at carbons next to the ring fusion predominates, as in the naphthalene system. Nucleophiles react preferentially at the electron-poor pyridine nucleus. These reactions are analogous to those with pyridine itself. Representative higher aza analogs of naphthalene: Alkaloids: Physiologically Potent Nitrogen Heterocycles in Nature 25-8 Alkaloids are bitter-tasting, natural, nitrogen-containing compounds found primarily in plants. Alkaloids often have potent physiological activity. Several common stimulants are in the alkaloid family. Nicotine: 2-8% in dried tobacco leaves Caffeine and theobromine: found in coffee and tea or cocoa Cocaine: extracted from the leaves of the coca shrub